Abstract
GABA-A receptors mediate both phasic synaptic inhibition and more recently appreciated tonic currents in the vertebrate central nervous system. We addressed discrepancies in the literature regarding the pharmacology of tonic currents by examining tonic currents in a controlled environment of dissociated, solitary glutamatergic neurons. We describe a novel tonically active, bicuculline-sensitive chloride conductance that is insensitive to gabazine and to picrotoxin and thus not mediated by conventional GABA receptors. We exclude a significant contribution from small conductance calcium-gated potassium (SK) channels. We also pharmacologically exclude calcium-gated chloride channels, glycine receptors and the chloride current associated with glutamate transport. Finally we demonstrate that although small, this current modulates neuronal excitability. We speculate that this tonic current may provide a complementary mechanism for the regulation of neuronal excitability, particularly in regions with low ambient GABA concentrations. We conclude that this bicuculline-sensitive conductance needs to be accounted for in studies of GABA tonic currents, lest it be confused with currents associated with GABA overflow.
Keywords: tonic current, chloride, inhibition, hippocampus, GABA
1. INTRODUCTION
Tonic, resting membrane conductances, such as those mediated by leak K+, Cl−, and transmitter-gated channels, strongly influence neuronal excitability. There is recent particular interest in tonic conductances mediated by GABA-A receptors, often defined pharmacologically [11]. GABA’s actions at GABA-A receptors underlie well-known phasic, synaptic actions [19,31] as well as more recently described tonic conductances [11]. GABA-A mediated tonic chloride conductances, although small in amplitude, can have a profound influence on neuronal excitability because the tonic current provides an ongoing shunt to voltage-gated currents [27]. Tonic currents are defined by the ability of GABA-site antagonists, like bicuculline or gabazine, to block a resting conductance. A tonic conductance has been reported in many neuronal types and is currently thought to arise primarily from GABA that accumulates at extrasynaptic sites during synaptic activity [25,39]. Nevertheless, there is controversy over whether the antagonist pharmacology of tonic conductances are distinct from those of phasic, synaptic conductances [1,33]. In some studies, tonic, but not phasic, currents have exhibited resistance to gabazine but sensitivity to bicuculline, two GABA-site antagonists [1]. It is possible that the pharmacology of antagonists is not always selective enough to truly identify GABA receptor mediated conductances.
Here we use a reduced, single-cell preparation to re-examine the pharmacology of tonic chloride conductances in hippocampal neurons. Like others [1], we find evidence for a bicuculline-sensitive, gabazine-insensitive tonic current. However, the tonic current exhibits a different pharmacology than the tonic current gated by low GABA concentrations or by neurosteroids, potential endogenous regulators of GABA-receptor mediated tonic currents. Notably, the current is insensitive to the non-competitive GABA-A receptor antagonist picrotoxin. Therefore, this current is not mediated by ambient GABA or by conventional GABA receptors. We conclude that this novel, bicuculline-sensitive chloride conductance could contribute to tonic inhibition under some conditions. Future studies of tonic currents should account for this conductance since it can easily be confused with currents mediated by GABA overflow.
2. Results
2.1 Tonic chloride conductance in solitary glutamatergic neurons
Figure 1A illustrates representative autaptic currents from solitary excitatory hippocampal neurons. Shown is the response in the absence of antagonist (baseline), in the presence of NBQX (1 μM) and in the presence of 1 μM NBQX plus 25 μM bicuculline. Despite the glutamate phenotype of this solitary neuron, addition of bicuculline decreased the baseline noise level and produced an outward shift in the holding current level (Fig. 1B) with no effect on the evoked autaptic current. Similar results were obtained in 5 other neurons. Although GABA release from glutamate neurons is reported from dentate granule neurons under some conditions (reviewed in [14]) and from cultured hippocampal neurons [2], the lack of effect of bicuculline on evoked responses argues against GABA release in our system and our subsequent pharmacology (see Section 2.3) further excludes an important role for GABA in the bicuculline-sensitive current.
Figure 1.
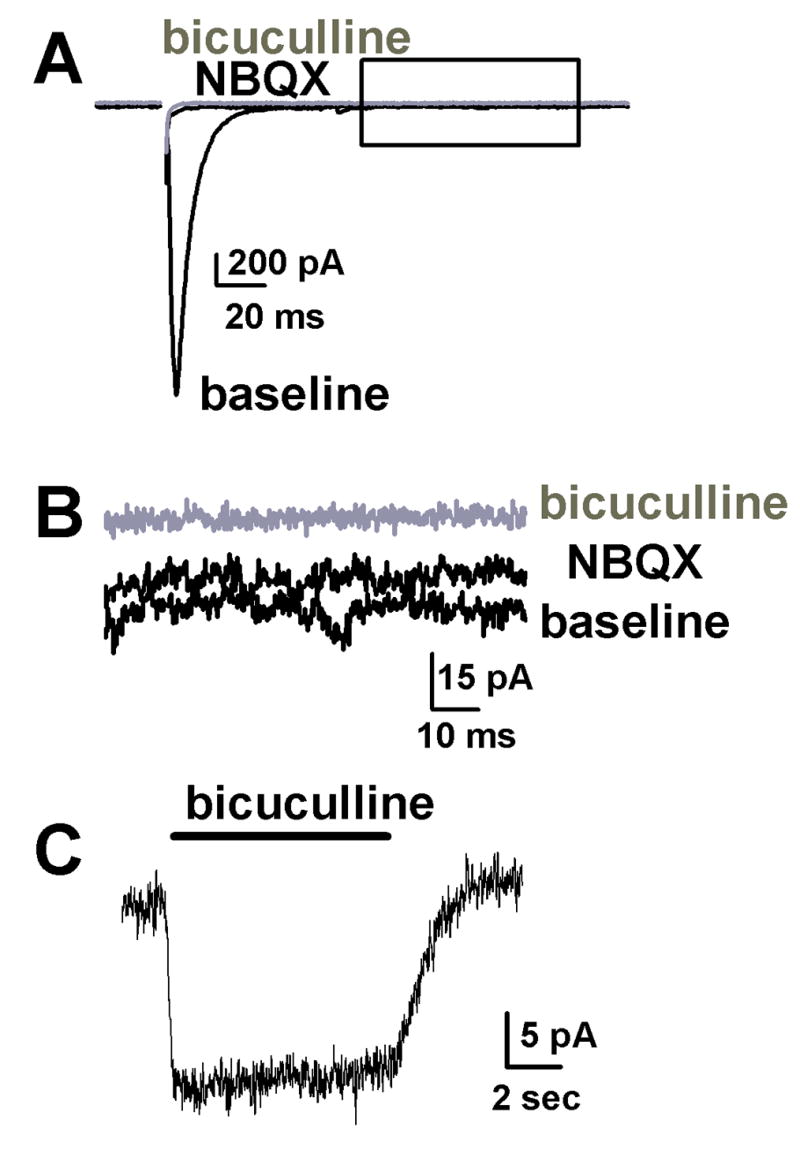
Tonic current in single glutamatergic hippocampal neurons is revealed by application of bicuculline. A. An autaptic EPSC is blocked by application of 1 μM NBQX (black traces). Addition of 25 μM bicuculline had no effect on the response to stimulation (grey trace). B. However as shown in the expanded view of the current marked by a box in panel A, bicuculline did cause decreased noise and an outward shift in the holding current. C. Application of 10 μM bicuculline again reveals a tonic chloride current in another neuron. This and subsequent experiments were performed using pipettes containing CsMeSO4 resulting in a more negative chloride reversal potential and a holding potential of −20 mV to provide adequate driving force.
A KCl-containing pipette solution was used for these experiments to promote the ability to detect any synaptically released GABA from glutamatergic cells [2,14] (negligible in Figure 1A). For subsequent experiments, we set the chloride equilibrium potential to more negative values by using a large anion (methanesulfonate) in the pipette solutions, so that we could readily distinguish glutamatergic from GABAergic cells on the basis of autaptic current polarity at negative membrane potentials. We also loaded the cells with cesium as the primary cation to block potassium conductances. Under these conditions we noted that in solitary glutamatergic neurons, a tonic, bicuculline-sensitive outward current was present at membrane potentials distant from the chloride equilibrium potential (Fig. 1C). Because of the small size of the current, it was not possible to obtain robust concentration-response data. However, this and subsequent experiments were performed using 10 μM bicuculline since higher concentrations of bicuculline did not have any additional effect. We found no change in the current density with the age of the cultures tested. The pooled average current density was 0.13 ± 0.022 pA/μF (n = 14), corresponding to an average whole-cell current of 8.6 ± 1.5 pA (n = 14). This amplitude is in line with that of studies of putatively GABA-mediated tonic currents [1,33].
We considered the possibility that the tonic current might be mediated by reverse transport of GABA [13] or another ligand from the neuron or from underlying astrocytes. If reverse uptake was responsible for the current, we would expect that replacement of extracellular sodium with choline should enhance the neuronal current, as these ionic conditions favor reverse uptake [13] Although net outward current was increased in neurons bathed in choline, the additional outward current was not sensitive to bicuculline (fig. 2A; N = 8, 6.9 ± 2.8 pA average bicuculline sensitive current in sodium bath versus 4.3 ± 1.1 pA average bicuculline sensitive current in choline bath, p > 0.19, paired T-Test), thereby excluding a role for reverse transport in generation of the bicuculline-sensitive currents.
Figure 2.
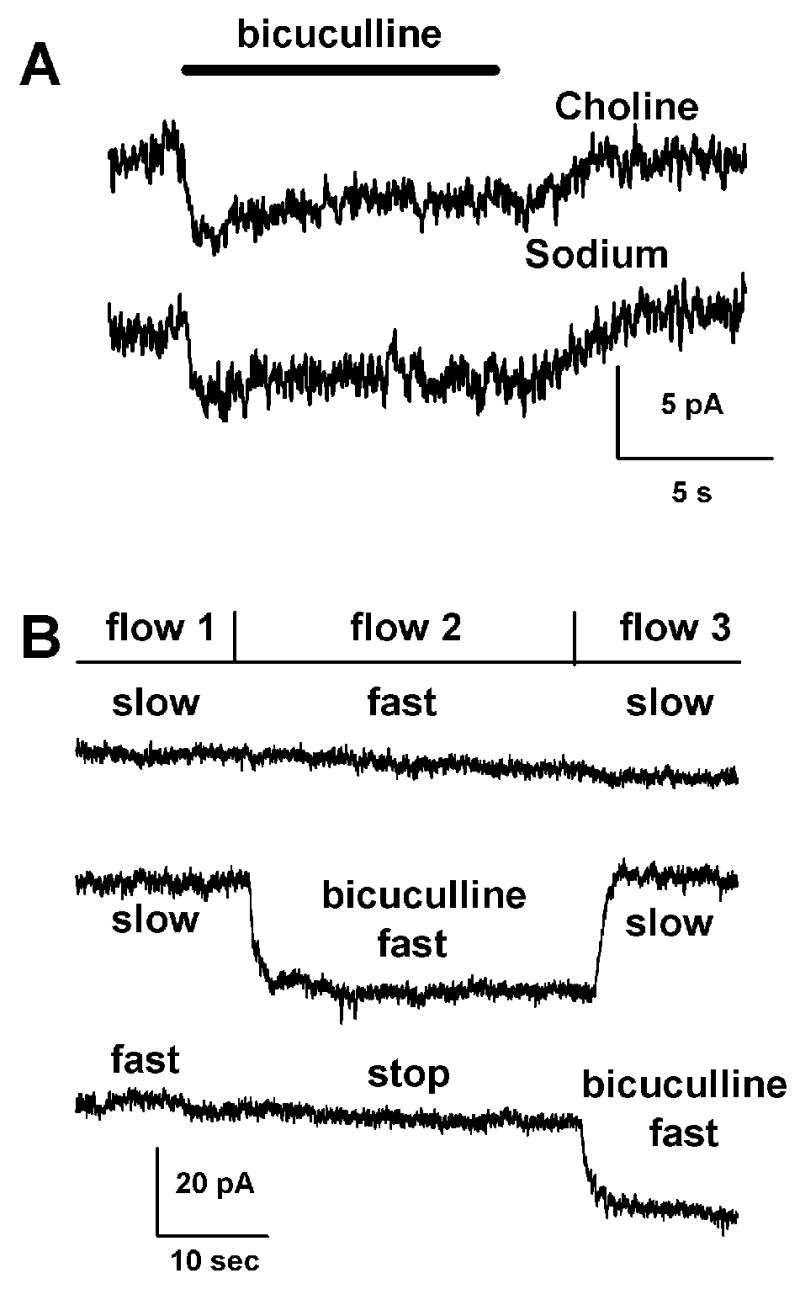
There is no evidence of an endogenous agonist mediating the tonic current. A. Replacing the sodium in the external bath solution had no measurable effect on the amplitude of the tonic current suggesting that reverse transport of GABA does not contribute to the current (n= 8). B. Similarly, the amplitude of the tonic current is not reduced by rapid solution flow nor increased by stopping flow, suggesting that the current is not mediated by tonic release of agonist (n= 11). Shown are three traces from a single neuron with the baselines of the second and third traces shifted for clarity. During each trace the cell was exposed to three solution flows as shown. Vm was −20 mV with a cesium methanesulfonate pipette solution.
Next, we tested for the presence of a continuously released ligand [18]. On glutamatergic islands, we have shown that tonic neuronal glutamate currents can be recorded under certain conditions, attributable to astrocyte reverse uptake of glutamate [20,23]. The amplitude of tonic glutamate currents is augmented by halting solution flow, allowing buildup of local ambient glutamate under conditions of a static bath. In contrast, bicuculline-sensitive currents were not augmented by static bath conditions (n = 11; Fig. 2B). Lack of effect of static bath conditions might be due to a saturating concentration of ligand. However, rapid flow of control solution also had no effect on the tonic current (Fig. 2B). Therefore, continuous release of a ligand appears unlikely.
2.2 SK channels do not mediate the tonic current
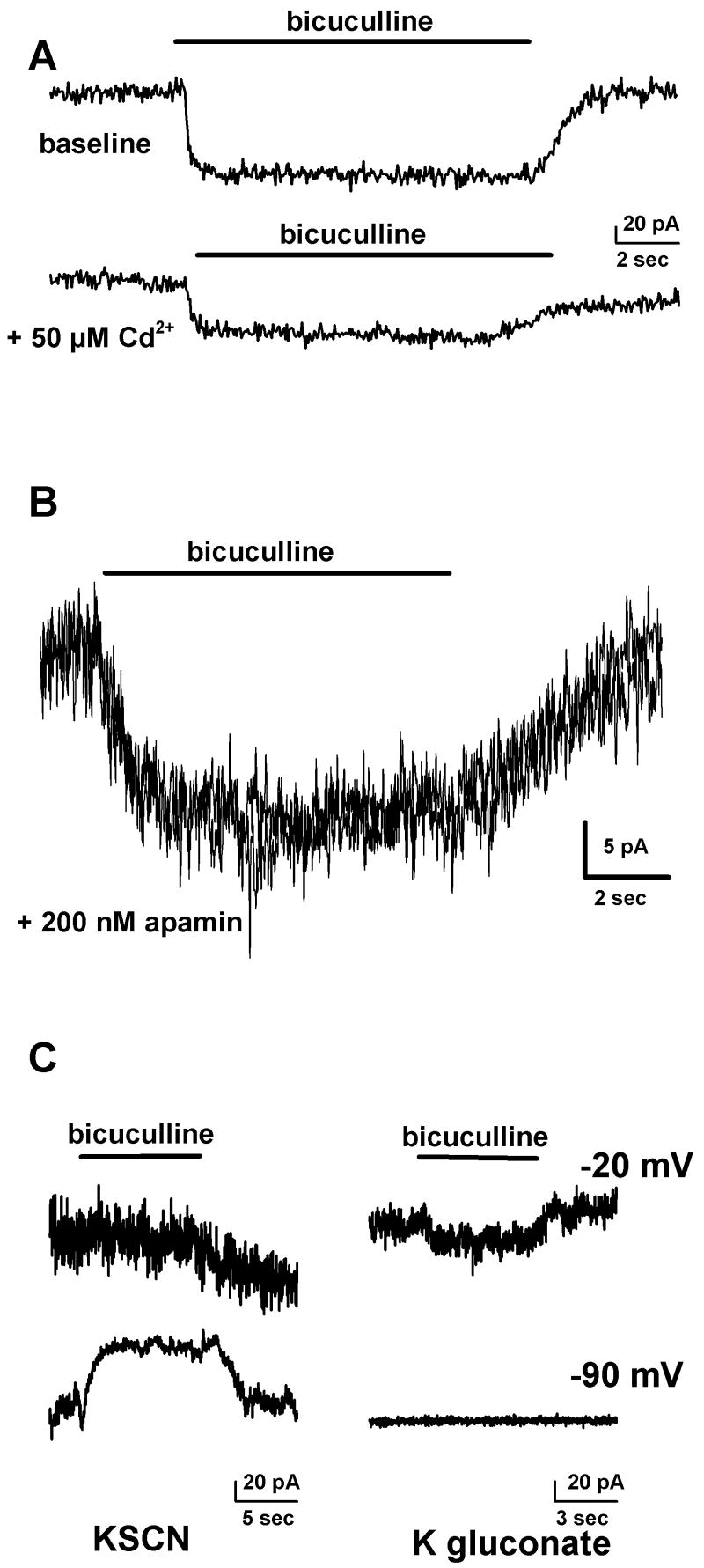
The lack of any evidence for a local source of GABA (or another agonist) raises the possibility that the tonic current is mediated by another channel. In particular, bicuculline is also known to block SK-type Ca2+-activated potassium conductances [8,17]. Although we filled cells with cesium to block potassium conductances, it is possible that a small fraction of unblocked current could account for the tonic current. However, the following observations exclude a contribution of SK current to the tonic currents. First, tonic currents were still observed in 50 μM Cd2+ to block Ca2+ influx and SK current activation (n = 9; Fig 3A). Second, bicuculline-sensitive current was still observed in 200 nM apamin, a selective, irreversible blocker of SK current (n = 7; Fig. 3B). Third, although it was difficult to obtain reversal potential measurements on tonic currents because of their small amplitude, filling cells with the highly permeant anion thiocyanate clearly increased the amplitude of inward bicuculline-sensitive tonic current at negative membrane potentials (Fig. 3C), consistent with the contribution of a chloride rather than a potassium conductance.
Figure 3.
The tonic current is not mediated by calcium-activated potassium (SK) channels. A. To test the alternative hypothesis that SK channels mediate the tonic current, currents were measured using 10 μM bicuculline in the absence and presence of 50 μM cadmium. The tonic current persisted in the presence of cadmium (n = 9). Vm was −20 mV with a cesium methanesulfonate pipette solution. B. The tonic current also persisted in the presence of the specific SK channel blocker apamin (200 nM; n = 7). Vm was −20 mV with a cesium methanesulfonate pipette solution. C. Measuring tonic currents using pipettes filled with the highly permeant anion thiocyanate (left panels) reveals an inward current that is more prominent at a more negative holding potential. In contrast, the tonic current measured using pipettes filled with the impermeant anion gluconate (right panels) is more prominent at a less negative holding potential. In both cases, the potassium reversal potential is near −70 mV while the chloride reversal potential is near 0 mV with thiocyanate and near −70 mV with gluconate. The response of the tonic current amplitudes to changing the anion in the recording pipette is most consistent with a chloride current.
2.3 Pharmacology
One explanation for the above results is that the tonic bicuculline sensitive current is mediated by spontaneous opening of GABA receptors in the absence of ligand [4]. We sought to explore this hypothesis by characterizing the pharmacology of the tonic current. We examined the ability of the GABA-site ligand, gabazine (10–30 μM), to inhibit the tonic current in solitary hippocampal neurons. We found that gabazine was ineffective in blocking bicuculline-sensitive tonic currents in solitary glutamatergic neurons (n = 7; Fig. 4A). On the other hand, tonic currents in GABAergic neurons were significantly inhibited by gabazine, consistent with the idea that ongoing autaptic release of GABA participates strongly in the tonic currents of solitary GABA cells (n = 4; Fig. 4B). Gabazine-insensitive tonic currents have been described previously [1] and could be consistent with either spontaneously opening channels [4] or with steroid-gated channels [30]. This is because bicuculline and gabazine are both allosteric modulators of GABA-A receptors [36]. Both inhibit channel activity by binding to the GABA site, even when the channel is opened by a GABA-less mechanism. Gabazine is a less effective allosteric modulator than bicuculline [7,35,36], thereby potentially accounting for relative gabazine insensitivity. If this explanation is correct, gabazine, although inert by itself, should occlude bicuculline’s ability to allosterically block the tonic current [1,35].
Figure 4.
The tonic current is not blocked by the competitive GABA antagonist gabazine. A. The response of a typical glutamatergic neuron to 10 μM bicuculline is illustrated in the initial part of the trace. In contrast, 30 μM gabazine has no effect on the tonic current in the same neuron while the combination of bicuculline and gabazine again blocks the tonic current. Note that while gabazine is ineffective alone, it does partially occlude the ability of bicuculline to block the tonic current as switching from the combination of gabazine and bicuculline to bicuculline alone results in an additional amount of block (average 37.3 ± 11.0%; n = 7). B. The same experiment was repeated in a GABAergic neuron. At the −20 mV holding potential, there is continual release of GABA as seen by the larger noise amplitude in the baseline current and the more pronounced block of that noise by the GABA antagonists. In this case both gabazine and bicuculline block the tonic current. Note the additional block that occurs with the addition of bicuculline to gabazine (average 30.1 ± 11.3%; n = 4). This portion of the current that is not blocked by gabazine represents the spontaneous tonic current in inhibitory neurons. C. One possible explanation for the lack of direct gabazine effect is that the tonic current is mediated by neurosteroids (see text for details). While gabazine is a poor antagonist of steroid mediated currents, it strongly interferes with bicuculline block of steroid gated currents. Gabazine partially blocked the current mediated by 0.3 μM 3α5αP and strongly occluded bicuculline block as illustrated by the increase in bicuculline block when gabazine was removed (n = 4). Note that gabazine was much more effective in occluding bicuculline block of steroid mediated currents (average 3.5 ± 1.3% additional block from the combination of gabazine and bicuculline) compared to the tonic current (average 76.6 ± 5.5% additional block from the combination). In all panels, holding potential is −20 mV with a cesium methanesulfonate pipette solution.
We tested this prediction and found that gabazine only partially occluded bicuculline’s ability to block tonic currents in excitatory hippocampal neurons (Fig. 4A). By contrast, gabazine was effective at blocking phasic IPSCs and associated GABAergic tonic currents in autaptic GABAergic neurons (Fig. 4B). However, in these cells, as in glutamatergic cells, there was a gabazine-insensitive, bicuculline-sensitive component of tonic current (Fig. 4B).
In excitatory neurons we examined the similarity of the tonic current pharmacology with that of currents gated by the neuroactive steroid (3α,5α)-3-Hydroxypregnan-20-one (3α5αP; allopregnanolone). As expected, saturating concentrations of gabazine only partially inhibited steroid-gated currents [30]. Gabazine blocked an average of 31.1 ± 7.4% (n = 4) of the 3α5αP mediated current. However, this partial inhibition was sufficient to nearly completely occlude bicuculline block of the 3α5αP mediated current. The combination of gabazine and bicuculline only blocked an additional 3.5 ± 1.3% of the 3α5αP mediated current (Fig 4C). In contrast, bicuculline plus gabazine blocked an average of 76.6 ± 5.5% (n = 7) of the tonic current (Fig 4A).
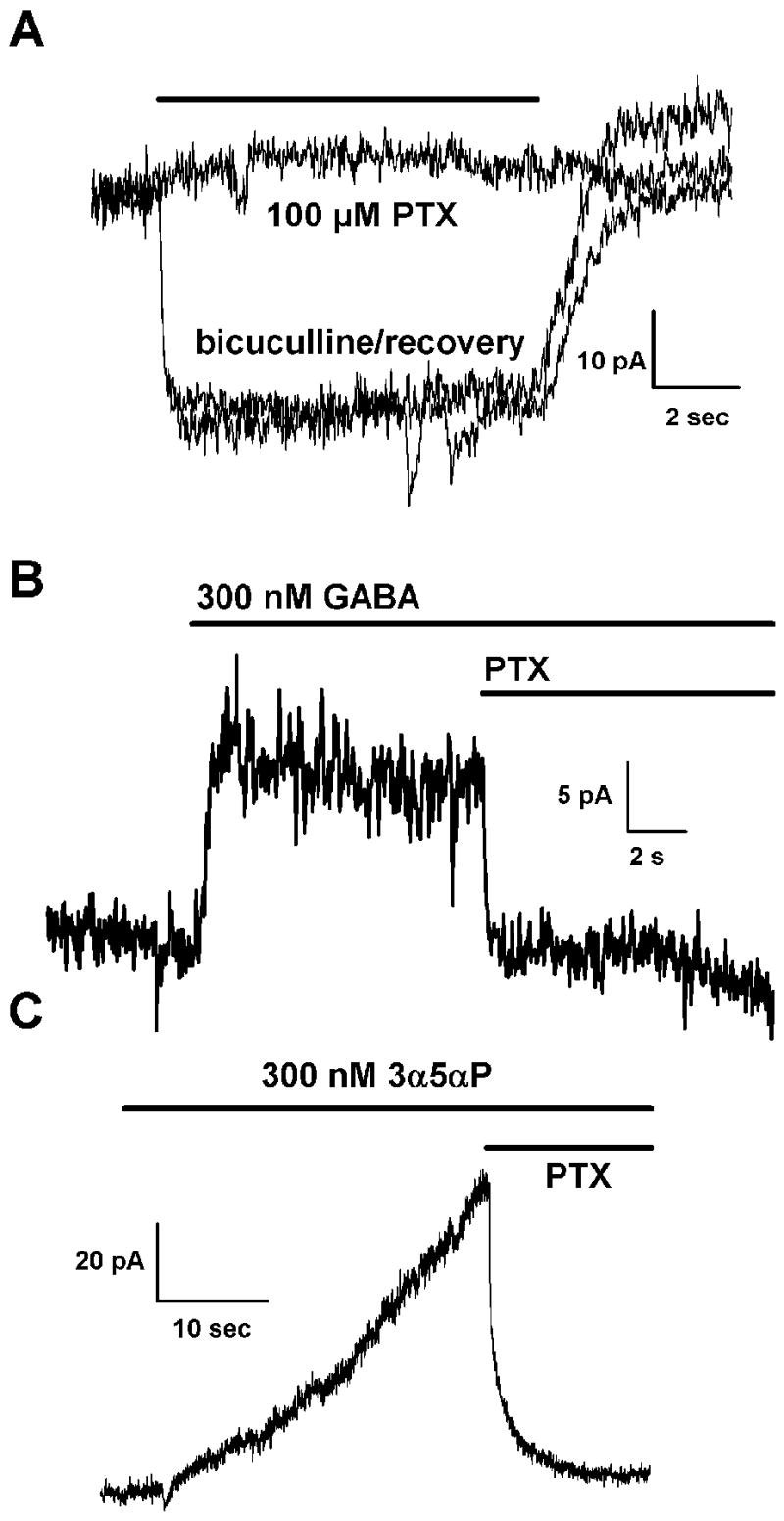
The quantitative discrepancy in gabazine occlusion of steroid currents versus spontaneous tonic currents makes it unlikely that endogenous steroid mediates the tonic current. To further test the likelihood that steroid or spontaneous gating of conventional GABA-A receptors mediates the current, we tested the non-competitive GABA-A receptor antagonist picrotoxin. We also found that picrotoxin did not affect tonic currents (n = 4; Fig. 5A). Channel openings resulting in tonic currents are presumably low probability events arising from a large population of channels. Therefore, although we used a high concentration of picrotoxin, the lack of picrotoxin effect could result from the activation dependence of picrotoxin block [9,40]. To test this, we examined the ability of picrotoxin (100 μM) to block the response to low GABA or steroid concentrations in solitary glutamatergic cells (Fig. 5B, C). We found that picrotoxin effectively blocked these responses, indicating the lack of picrotoxin effect did not result from the low probability of opening of tonic-current channels. We conclude from this picrotoxin insensitivity that spontaneous or steroid gating of conventional GABA-A receptors does not underlie the bicuculline-sensitive tonic current.
Figure 5.
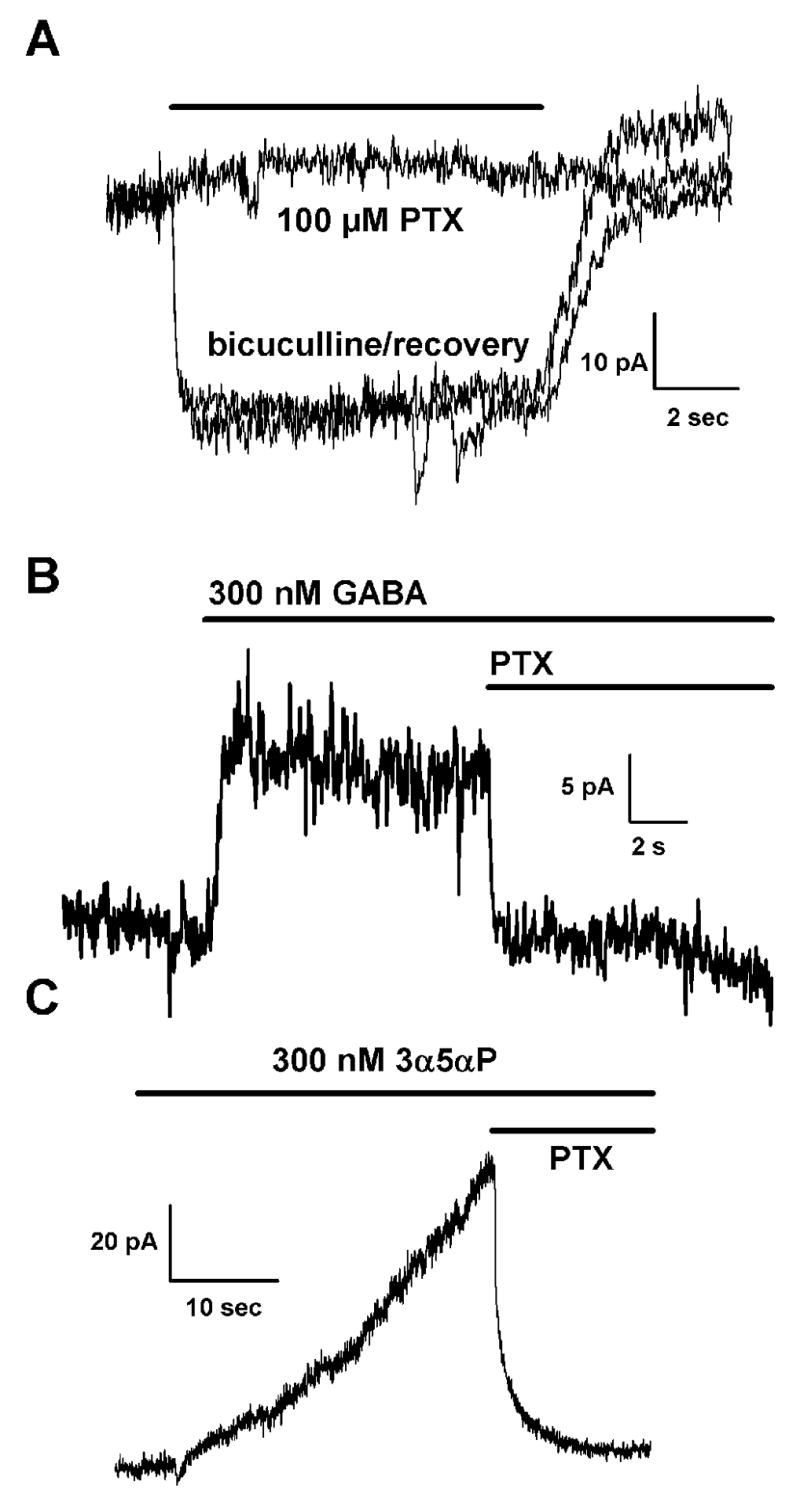
The tonic current is not blocked by picrotoxin. A. 100 μM picrotoxin had no effect on the tonic current (n = 4). Control sweeps with 10 μM bicuculline were made prior to and after the picrotoxin application. In contrast, picrotoxin effectively blocked currents mediated by 0.3 μM GABA (panel B) and 0.3 μM 3α5αP (panel C), again suggesting that neither GABA nor steroids mediate the tonic current. Vm was −20 mV with a cesium methanesulfonate pipette solution for all panels.
Recently, there have been several reports of GABA receptors with atypical pharmacology [15,24,26,28]. These receptors are currently thought to include some combination of typical GABA-A receptor subunits (alpha, beta and/or gamma) and GABA-C receptor rho subunits [15,24]. Some of these receptors are characterized by sensitivity to bicuculline and resistance to picrotoxin [26,28]. They are also sensitive to the GABA-C antagonist (1,2,5,6-tetrahydropyridin-4-yl)methlyphosphinic acid (TPMPA) [15,24]. In contrast, the tonic current in our system was not affected by TPMPA at concentrations up to 500 mM, which is sufficient to partially block GABA-A receptors [16] and sufficient to depress evoked IPSCs in our GABAergic neurons (data not shown).
2.4 Other chloride channels do not mediate the tonic current
We next considered the possibility of other chloride conductances mediating the tonic current. The first possibility we considered is that the tonic current is mediated by glycine receptors. However, application of 500 nM of the glycine antagonist strychinine, which is a saturating concentration in cultured hippocampal neurons [34], had no effect on the tonic current (data not shown). We next considered the possibility that the tonic current is mediated by a calcium-gated chloride current. This hypothesis was tested by application of 100 μM niflumic acid, a blocker of many calcium gated chloride currents [reviewed in 12]. Again, there was no effect on the tonic current in hippocampal neurons (data not shown). Finally we considered the possibility that the tonic current is mediated by a chloride current associated with excitatory amino acid transport [10,37]. We applied 50 μM of the excitatory amino acid transporter blocker DL-threo-β-benzyloxyaspartate (TBOA) [29], which had no effect on the tonic current (data not shown).
2.5 Physiological relevance
The data from these experiments suggest the contribution of a novel chloride conductance to tonic currents in cultured hippocampal neurons. Is this conductance relevant to cellular excitability, or is it too small to significantly affect the active properties of neurons? To address this question, we examined excitability of solitary glutamatergic cells in the presence and absence of bicuculline. Except to initially define the synaptic phenotype of the recorded cells, postsynaptic blockers (NBQX, D-APV) were present in the bath solution so that autaptic interactions did not affect excitability. We adjusted the level of a 6–8 s depolarizing current injection to elicit a train of spikes in the baseline saline. Then we repeated the same current injection in the presence of 10 μM bicuculline. Resting potential was set to −65 mV. There was no detectable effect of bicuculline on the resting potential. Figure 6A shows a representative example from 12 cells. We found that bicuculline reliably and reversibly increased the average frequency of spiking during the depolarizing current injection. Figure 6B summarizes the results from 12 neurons. In 5 of the neurons, 100 nM apamin was present in the bath (Fig. 6A represents a cell from this subset) to test for the possibility that SK-type Ca2+ -dependent K+ conductances mediate the effect of bicuculline on spiking pattern in these conditions. There was no notable difference in spiking patterns, and no difference in bicuculline effects in this subset, consistent with the idea that SK-type Ca2+ -dependent K+ conductances are not important in the bicuculline effects.
Figure 6.
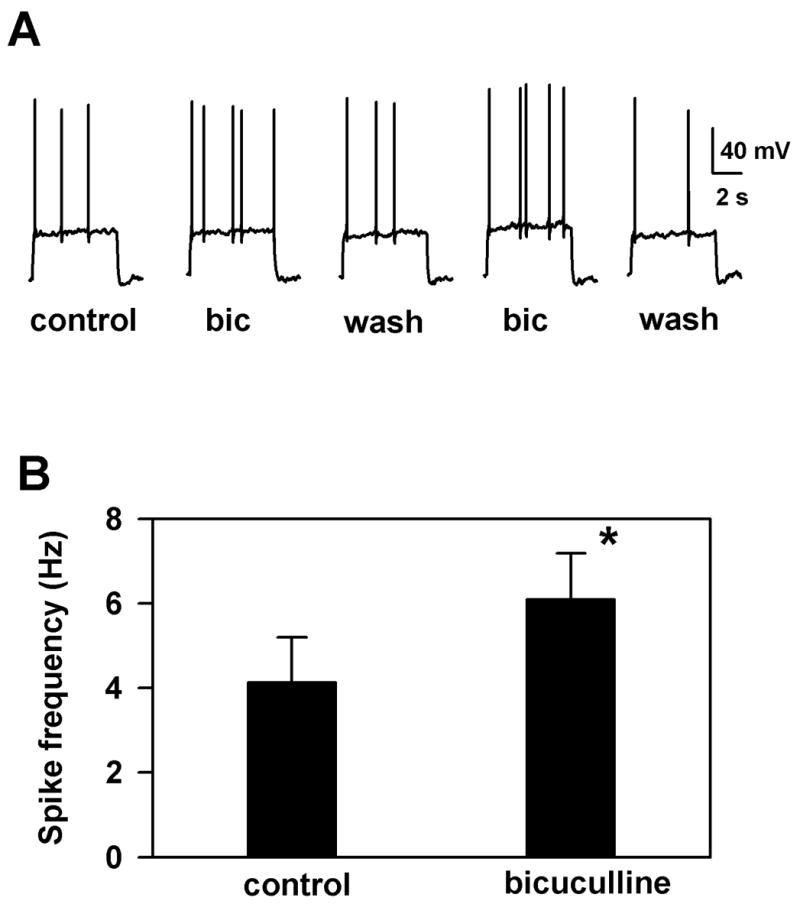
The tonic current can modulate neuronal firing frequency. A. Application of 10 μM bicuculline increases the firing frequency elicited by current injection. In this cell, 100 nM apamin was also present. Responses were recorded using pipettes containing potassium gluconate. Resting potential was −65 mV. B. Data from 12 cells (including 5 tested in the presence of apamin) are summarized in the bar graph. There is a statistically significant increase of the firing rate in the presence of bicuculline (p = 0.01, N = 12, paired t-test). Error bars represent standard error of the mean.
3. Discussion
This work describes a spontaneous, bicuculline-sensitive chloride conductance in solitary cultured hippocampal neurons. Sensitivity to bicuculline and partial occlusion of bicuculline block by gabazine shows some similarity of this tonic conductance with currents mediated by conventional GABA-A receptors. However, manipulation of GABA transport and alteration of solution flow rates had no effect on the tonic current. This finding argues against an endogenous agonist-mediated conventional GABA-A receptor current, although a response mediated by GABA receptors saturated by agonist cannot be completely excluded. The complete lack of effect of gabazine is also not consistent with an agonist activated conventional GABA-A receptor current. Gabazine is a potent competitive antagonist of GABA at conventional GABA-A receptors, so the lack of response to gabazine argues against a current mediated by endogenous GABA. Because gabazine is also an allosteric inhibitor of other agonists at GABA-A receptors (see below), the lack of effect of gabazine argues against another endogenous agonist activating conventional GABA-A receptors. We have previously demonstrated that the neuroactive steroid antagonists (3α,5α)-17-phenylandrost-16-en-3-ol (17-PA) and γ-cyclodextrin have no direct effect on GABA-A receptors expressed in cultured hippocampal neurons and do not alter the tonic chloride conductance in these cells [21,30]. Additionally, the use of Cd2+ in experiments testing for the presence of small conductance calcium-gated potassium (SK) channels (see below) should also have blocked any residual Ca2+ dependent synaptic release of GABA. However, Cd2+ failed to block the tonic current.
The tonic current, unlike currents gated by low concentrations of exogenous GABA or by neurosteroids, is also insensitive to picrotoxin. This result alone is strong evidence that the tonic conductance is not mediated by conventional GABA receptors capable of being gated by GABA. Although picrotoxin-insensitive GABA receptors containing both GABA-A and GABA-C subunits have been described [26,28], the lack of effect of the GABA-C antagonist TPMPA and the ability of picrotoxin to completely block the current mediated by exogenous GABA argue against the presence of such receptors. Collectively, these data are inconsistent with agonist-mediated activation of conventional GABA-A receptors and strongly suggest that the tonic current does not result from either a conventional GABA-A conductance or any of the previously described GABA receptors containing both GABA-A and GABA-C subunits. We cannot fully exclude that the tonic conductance is generated by atypical or mis-assembled subunits capable of generating a spontaneous current that is insensitive to gabazine and picrotoxin.
One alternative hypothesis is that bicuculline is blocking a small conductance calcium-gated potassium (SK) channel [8,17]. However, neither the use of cadmium to block calcium channels nor the inclusion of the SK channel blocker apamin in the bath eliminated the tonic current. Furthermore, an increased current in the presence of highly permeant intracellular thiocyanate anions strongly suggests that the tonic current is mediated by a chloride conductance rather than a potassium conductance. Although many experiments account for the bicuculline sensitivity of SK channels by recording under conditions where SK channels are not activated, our results indicate that additional caution is warranted and that other conductances can easily be confused with those activated by GABA.
As noted above, the tonic current is not blocked by the GABA-A antagonist gabazine. Gabazine and bicuculline both act as allosteric inhibitors of non-GABA mediated activation of GABA-A receptor [36], presumably by binding to the GABA binding site and preventing transition to an open state. Gabazine insensitivity might result from the lower efficacy of gabazine as an allosteric inhibitor [7,35,36]. Despite its lower efficacy as an allosteric inhibitor, gabazine binds strongly enough to the GABA binding site to successfully compete with bicuculline binding and thereby occlude allosteric inhibition of GABA-A receptors by bicuculline [1,35]. As shown in Figure 4, gabazine only weakly protected the tonic current from block by bicuculline. These results differ somewhat from those of Bai et al. [1], who found that tonic currents were gabazine insensitive but that gabazine significantly occluded bicuculline’s effects.
Although the bicuculline-sensitive tonic current is relatively small, it clearly modulates excitability as demonstrated by the effect of bicuculline on responses to current injection. It is possible that the current has been overlooked because of experimental conditions in previous experiments. Tonic GABA currents are usually studied under conditions of ambient GABA presence, often achieved by boosting GABA levels with transporter or transaminase inhibition [e.g. 6,33] or with the direct addition of low concentrations of GABA to the extracellular solution [e.g. 38]. Furthermore, gabazine is often used to define tonic currents [32,38], which would preclude detection of the gabazine-insensitive tonic current characterized here. Although the gabazine-insensitive tonic current could represent a compensatory response of solitary cells to lack of GABA stimulation [5], we found evidence for gabazine insensitive tonic currents in many solitary GABAergic neurons as well. Thus, spontaneously active chloride currents may provide a complementary mechanism for the regulation of neuronal excitability in vivo, and may provide an additional mechanism by which bicuculline can induce epileptiform activity.
In summary, we report a novel, spontaneously active, bicuculline sensitive tonic chloride conductance in hippocampal neurons. This current may provide a mechanism complementary to GABA-gated condutances for the regulation of neuronal excitability, particularly in regions with low ambient GABA concentrations. We urge caution in interpreting bicuculline-sensitive tonic chloride currents as GABA-mediated currents.
4. Experimental Procedure
4.1 Hippocampal microcultures
Primary microcultures of hippocampal cells were prepared from 1 to 3 day postnatal Sprague Dawley rats as previously described [22]. Halothane anesthetized rats were decapitated and the hippocampi removed. Hippocampi were cut into 500 μm-thick transverse slices. Single-cell suspensions were prepared with 1 mg/ml papain digestion in oxygenated Leibovitz L-15 medium, followed by mechanical trituration in modified Eagle’s medium containing 5% horse serum, 5% fetal calf serum, 17 mM D-glucose, 400 μM glutamine, 50 U/ml penicillin, and 50 μg/ml streptomycin. Cells were plated in the modified Eagle’s medium at a density of 75 cells/mm2 onto 35 mm plastic culture dishes pre-coated with collagen microdroplets sprayed on a layer of 0.15% agarose. The anti-mitotic cytosine arabinoside (5–10 μM) was added on the third day after plating to halt glial proliferation. Electrophysiology was performed 12–18 days following plating.
4.2 Culture electrophysiology
Whole-cell recordings were performed on hippocampal microculture neurons, using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA) interfaced to a Pentium III-based computer via a Digidata 1322 acquisition board (Axon Instruments). At the time of experiments, culture medium was replaced with an extracellular recording solution consisting of (in mM): 138 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, 0.010 D-APV (pH 7.25). Recordings were at room temperature. Access resistance (3-10 MΩ) was electronically compensated 90–100% for synaptic currents. For all other currents, which were much smaller in amplitude, no access resistance compensation was performed. The standard pipette solution contained (in mM): 140 cesium methanesulfonate, 4 NaCl, 0.5 CaCl2, 5 EGTA, 10 HEPES (pH 7.25). For synaptic currents in Figure 1A, KCl was substituted for cesium methanesulfonate. For the reversal potential experiments in Figure 6C, cesium methanesulfonate was replaced with potassium thiocyanate or potassium gluconate. For the measurements of firing frequency in Figure 7A, cesium methanesulfonate was again replaced with potassium gluconate. Exogenous drugs were applied with a multi-barrel pipette coupled to miniature solenoid valves that allowed rapid switching (~100 ms on whole cells). For the experiments depicted in Figures 2–6, 1μM 1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide (NBQX) was included in all barrels. Slow and rapid solution flow rates were achieved by using small and large diameter tubing, respectively. Autaptic release of neurotransmitter was evoked in voltage-clamped solitary neurons with a 2 ms voltage pulse to 0 mV from a holding potential of −70 mV [3,22].
4.3 Data analysis
Data acquisition was performed with pCLAMP 9.0 software (Axon Instruments, CA). Data analysis was performed using pCLAMP, Igor (Wavemetrics, Lake Oswego, OR) and Excel (Microsoft, Redmond, WA). Data plotting and curve fitting were done with Sigma Plot 8.0 software (SPSS Science, Chicago, IL). Data are presented in the text and figures as mean ± standard error of the mean. Statistical differences were determined using a two-tailed Student’s t-test with a p-value less than 0.05 considered significant.
4.4 Drugs
All drugs were from Sigma (St. Louis MO). Bicuculline methobromide was used for all experiments.
Acknowledgments
This work was supported a gift from the Bantly Foundation (C.F.Z.), and NIH grants NS44041 (L.N.E), GM 47969 (C.F.Z.), AA12951 (C.F.Z.), NS40488 (S.M.), and AA12952 (S.M.).
ABBREVIATIONS
- 17-PA
(3α,5α)-17-phenylandrost-16-en-3-ol
- 3α5αP (allopregnanolone)
(3α,5α)-3-Hydroxypregnan-20-one
- D-APV
D( )-2-Amino-5-phosphonopentanoic acid
- EGTA
Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- GABA
γ-Aminobutyric acid
- HEPES
N-(2-Hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid) hemisodium salt
- NBQX
1,2,3,4-tetrahydro-6-nitro-2,3-dioxobenzo[f]quinoxaline-7-sulfonamide
- TBOA
DL-threo-β-benzyloxyaspartate
- TPMPA
(1,2,5,6-tetrahydropyridin-4-yl)methlyphosphinic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–24. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- 2.Bekkers JM. Presynaptically silent GABA synapses in hippocampus. J Neurosci. 2005;25:4031–9. doi: 10.1523/JNEUROSCI.4969-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekkers JM, Stevens CF. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci U S A. 1991;88:7834–8. doi: 10.1073/pnas.88.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birnir B, Everitt AB, Lim MS, Gage PW. Spontaneously opening GABA(A) channels in CA1 pyramidal neurones of rat hippocampus. J Membr Biol. 2000;174:21–9. doi: 10.1007/s002320001028. [DOI] [PubMed] [Google Scholar]
- 5.Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- 6.Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–7. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang Y, Weiss DS. Allosteric activation mechanism of the alpha1beta2gamma2 gamma-aminobutyric acid type A receptor revealed by mutation of the conserved M2 leucine. Biophys J. 1999;77:2542–51. doi: 10.1016/s0006-3495(99)77089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Debarbieux F, Brunton J, Charpak S. Effect of bicuculline on thalamic activity: a direct blockade of IAHP in reticularis neurons. J Neurophysiol. 1998;79:2911–8. doi: 10.1152/jn.1998.79.6.2911. [DOI] [PubMed] [Google Scholar]
- 9.Eisenman LN, He Y, Fields C, Zorumski CF, Mennerick S. Activation-dependent properties of pregnenolone sulfate inhibition of GABAA receptor-mediated current. J Physiol. 2003;550:679–91. doi: 10.1113/jphysiol.2003.043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 11.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 12.Frings S, Reuter D, Kleene SJ. Neuronal Ca2+ -activated Cl- channels--homing in on an elusive channel species. Prog Neurobiol. 2000;60:247–89. doi: 10.1016/s0301-0082(99)00027-1. [DOI] [PubMed] [Google Scholar]
- 13.Gaspary HL, Wang W, Richerson GB. Carrier-mediated GABA release activates GABA receptors on hippocampal neurons. J Neurophysiol. 1998;80:270–81. doi: 10.1152/jn.1998.80.1.270. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez R. The dual glutamatergic-GABAergic phenotype of hippocampal granule cells. Trends Neurosci. 2005;28:297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann K, Stief F, Draguhn A, Frahm C. Ionotropic GABA receptors with mixed pharmacological properties of GABAA and GABAC receptors. Eur J Pharmacol. 2004;497:139–46. doi: 10.1016/j.ejphar.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 16.Jones MV, Jonas P, Sahara Y, Westbrook GL. Microscopic kinetics and energetics distinguish GABA(A) receptor agonists from antagonists. Biophys J. 2001;81:2660–70. doi: 10.1016/S0006-3495(01)75909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khawaled R, Bruening-Wright A, Adelman JP, Maylie J. Bicuculline block of small-conductance calcium-activated potassium channels. Pflugers Arch. 1999;438:314–21. doi: 10.1007/s004240050915. [DOI] [PubMed] [Google Scholar]
- 18.Liu QY, Schaffner AE, Chang YH, Maric D, Barker JL. Persistent activation of GABA(A) receptor/Cl(-) channels by astrocyte- derived GABA in cultured embryonic rat hippocampal neurons. J Neurophysiol. 2000;84:1392–403. doi: 10.1152/jn.2000.84.3.1392. [DOI] [PubMed] [Google Scholar]
- 19.Mehta AK, Ticku MK. An update on GABAA receptors. Brain Res Brain Res Rev. 1999;29:196–217. doi: 10.1016/s0165-0173(98)00052-6. [DOI] [PubMed] [Google Scholar]
- 20.Mennerick S, Dhond RP, Benz A, Xu W, Rothstein JD, Danbolt NC, Isenberg KE, Zorumski CF. Neuronal expression of the glutamate transporter GLT-1 in hippocampal microcultures. J Neurosci. 1998;18:4490–9. doi: 10.1523/JNEUROSCI.18-12-04490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mennerick S, He Y, Jiang X, Manion BD, Wang M, Shute A, Benz A, Evers AS, Covey DF, Zorumski CF. Selective antagonism of 5alpha-reduced neurosteroid effects at GABA(A) receptors. Mol Pharmacol. 2004;65:1191–7. doi: 10.1124/mol.65.5.1191. [DOI] [PubMed] [Google Scholar]
- 22.Mennerick S, Que J, Benz A, Zorumski CF. Passive and synaptic properties of hippocampal neurons grown in microcultures and in mass cultures. J Neurophysiol. 1995;73:320–32. doi: 10.1152/jn.1995.73.1.320. [DOI] [PubMed] [Google Scholar]
- 23.Mennerick S, Shen W, Xu W, Benz A, Tanaka K, Shimamoto K, Isenberg KE, Krause JE, Zorumski CF. Substrate turnover by transporters curtails synaptic glutamate transients. J Neurosci. 1999;19:9242–51. doi: 10.1523/JNEUROSCI.19-21-09242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milligan CJ, Buckley NJ, Garret M, Deuchars J, Deuchars SA. Evidence for inhibition mediated by coassembly of GABAA and GABAC receptor subunits in native central neurons. J Neurosci. 2004;24:7241–50. doi: 10.1523/JNEUROSCI.1979-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rossi DJ, Hamann M. Spillover-mediated transmission at inhibitory synapses promoted by high affinity alpha6 subunit GABA(A) receptors and glomerular geometry. Neuron. 1998;20:783–95. doi: 10.1016/s0896-6273(00)81016-8. [DOI] [PubMed] [Google Scholar]
- 26.Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–90. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- 27.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–9. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Shen DW, Higgs MH, Salvay D, Olney JW, Lukasiewicz PD, Romano C. Morphological and electrophysiological evidence for an ionotropic GABA receptor of novel pharmacology. J Neurophysiol. 2002;87:250–6. doi: 10.1152/jn.00620.2001. [DOI] [PubMed] [Google Scholar]
- 29.Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 30.Shu HJ, Eisenman LN, Jinadasa D, Covey DF, Zorumski CF, Mennerick S. Slow actions of neuroactive steroids at GABAA receptors. J Neurosci. 2004;24:6667–75. doi: 10.1523/JNEUROSCI.1399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 32.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–44. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stell BM, Mody I. Receptors with different affinities mediate phasic and tonic GABA(A) conductances in hippocampal neurons. J Neurosci. 2002;22:RC223. doi: 10.1523/JNEUROSCI.22-10-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thio LL, Shanmugam A, Isenberg K, Yamada K. Benzodiazepines block alpha2-containing inhibitory glycine receptors in embryonic mouse hippocampal neurons. J Neurophysiol. 2003;90:89–99. doi: 10.1152/jn.00612.2002. [DOI] [PubMed] [Google Scholar]
- 35.Thompson SA, Smith MZ, Wingrove PB, Whiting PJ, Wafford KA. Mutation at the putative GABA(A) ion-channel gate reveals changes in allosteric modulation. Br J Pharmacol. 1999;127:1349–58. doi: 10.1038/sj.bjp.0702687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci. 1997;17:625–34. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–8. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 38.Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–82. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–61. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon KW, Covey DF, Rothman SM. Multiple mechanisms of picrotoxin block of GABA-induced currents in rat hippocampal neurons. J Physiol. 1993;464:423–39. doi: 10.1113/jphysiol.1993.sp019643. [DOI] [PMC free article] [PubMed] [Google Scholar]