Abstract
DNA plasmids and recombinant adenovirus serotype-5 (rAd5) vectors are being studied in human clinical trials as HIV-1 vaccine candidates. Each elicits robust T-cell responses and modest antibody levels. Since protein immunization alone elicits antibody but not CD8 T-cell responses, we studied protein boosting of DNA and rAd5 HIV-1 vaccine vectors. A single Env protein immunization provided a marked boost in antibody titer in guinea pigs primed with either DNA or rAd5 vaccines, and the resulting antibody binding and neutralization levels were similar to those attained after thee sequential protein immunizations. Since both T-cell immunity and neutralizing antibodies are thought to be required for protection against HIV-1, it may be possible to establish a balanced T-cell and antibody response with appropriate vectored vaccines and improve the neutralizing antibody titer with protein boosting.
Keywords: HIV, neutralizing antibodies, adjuvant
I. Introduction
The continued spread of the human immunodeficiency virus type 1 (HIV-1) worldwide highlights the need for an effective vaccine. Based on studies of HIV-1 infection in humans, and SIV infection of macaques, it is believed that CD8 T-cells play an important role in control of viral replication [1]. It is hoped that vaccine elicited T-cell responses will be able to limit viral replication and prevent HIV-1 associated disease progression in those individuals who become infected. However, antibodies may be required to prevent the acquisition of HIV-1 infection. Neutralizing antibodies can block viral entry into human T-cells in vitro and can protect non-human primates from a chimeric simian-human immunodeficiency virus (SHIV) infection [2, 3]. Thus, a major goal is to develop an HIV-1 vaccine strategy that will generate both cellular immunity and high titers of virus neutralizing antibodies. Serial immunization with HIV-1 envelope (Env) protein immunogens can result in high antibody levels, but little or no CD8 T-cell responses [4–9]. One approach to generating both cellular immunity and neutralizing antibodies is to initiate immunization with a gene based vector that is known to elicit CD4 and CD8 responses, and boost with a protein immunogen.
Current gene-based immunogens, such as plasmid DNA and rAd5, have been widely studied in animal models [10–15], and have entered phase I and II human testing [16–19]. In addition, numerous groups have utilized vector prime, protein boost regimens to improve the potency or durability of the immune response to HIV or SIV antigens [20–30]. In this study, we evaluated the ability of our existing clinical vaccine products, multigene plasmid DNA and rAd5, to prime for a booster immunization with a soluble oligomeric Env protein. The plasmid DNA and rAd5 vectors used here were the same as those currently being studied in human trials [18, 19]. The DNA product consists of six individual plasmids. Three encode gp145 Env proteins (clade A, B, and C); the remaining three encode Gag, Pol and Nef. The rAd5 product contains 4 separate rAd5 vectors.
Three encode gp140 versions of the same Env proteins as above; a fourth encodes a Gag-Pol fusion protein. Data from non-human primate studies show that a series of three DNA immunizations, or a single rAd5 immunization, generates robust antigen specific CD4 and CD8 responses and moderate antibody levels [31, 32]. Recent phase I studies in humans have confirmed these results [18, 19]. Additionally, the combination of DNA prime, rAd5 boost has been extensively studied in small animals and non-human primates, and results in robust cellular immunity and high antibody levels [13, 32–37]. However, we have not systematically studied the ability of these DNA or rAd5 vaccine candidates to prime for a protein boost. Thus, these studies focus on the anti-Env response elicited by DNA or rAd5, and boosted with a soluble Env protein.
A soluble oligomeric gp140 Env protein based on the SF162 strain, and containing a deletion of the second variable region (V2), has been studied in animal models and is currently being tested in phase I human trials. Immunization with the dV2gp140 protein generates high titer antibody levels including homologous neutralizing antibodies [6, 38–41]. In addition, dV2gp140 elicits high antibody titers in monkeys primed with a DNA plasmids encoding the homologous env gene [42, 43]. To further asses the potential for protein boosting of both DNA and rAd5, we formulated the dV2gp140 Env protein with the MF59 adjuvant alone, or with one of two different classes of a synthetic oligodeoxynucleotide (ODN) containing unmethylated deoxycytosine-doexyguanosine (CpG) motifs. GpG ODN are Toll-like receptor 9 (TLR9) ligands that stimulate antigen presenting dendritic cells (DC) [44]. Class A ODN stimulate high levels of INFα from plasmactyoid DC; B-class ODN induce strong B-cell and NK cell activation and the more recently discover C-class ODN appear have properties of both the A- and B-class ODN [45, 46]. Since several ODN adjuvants are in phase I human testing, we evaluated if B and C-class ODN, when formulated with protein in the boost phase of immunization, could augment the antibody response elicited by MF59 alone.
The guinea pig (GP) studies described here were designed to evaluate anti-Env antibody responses elicited by combinations of existing vaccine immunogens that have advanced into human clinical trails. Current HIV-1 DNA and rAd5 vectors are known to elicit robust T-cell responses in humans, but rather modest antibody levels. The soluble dV2gp140 protein elicits high antibody levels, but not CD8 T-cell responses. Thus, in human trials, it might be possible to establish a CD4 and CD8 T-cell response with the appropriate vaccine vectors, and further boost the antibody response with an Env protein. In these GP studies, we observed that a series of three DNA immunizations, or a single rAd5 immunization, produced moderate levels of anti-Env antibody, and effectively primed for boosting with soluble protein. A single protein boost immunization resulted in high antibody levels and the induction of virus neutralizing antibodies. The addition of a B- or C-class ODN to the protein formulation in the boost phase of immunization did not augment the antibody response generated by MF59 alone. While current Env based immunogens do not readily generate broadly reactive neutralizing antibodies, theses data suggest that DNA plus protein, or rAd5 plus protein, are valuable vaccine platforms that can be used to study the generation of HIV-1 anti-Env antibody responses. In addition, it may be possible to establish a T-cell response with appropriate vectored vaccines and further improve the neutralizing antibody titer with protein boosting.
2. Materials and methods
2.1 Vaccine constructs
The plasmid DNA vaccine consisted of six individual plasmids each encoding a single HIV-1 gene product. HIV-1 clade B Gag, Pol, and Nef, and a clade A, B and C gp145 env, were each cloned into the expression vector CMV/R containing the human cytomegalovirus immediate early enhancer, promoter and intron region from HTLV-1 [47]. The gene inserts were synthetic codon optimized sequences and have been described previously [48]. The gag gene was derived from the clade B strain HXB2 (GenBank accession number K03455), the pol gene from clade B strain NL4-3 (GenBank accession number M19921) and the nef gene from the clade B strain PV22 (GenBank accession number K02083). The gp145 env gene inserts were contained the dCFI modifications as previously described [49]. The three env genes were derived from the clade A strain 92RW020 (CCR5-tropic, GenBank accession number U08794), the clade C strain 97Z012 (CCR5-tropic, GenBank accession number AF286227) and the clade B strain HxB2 (X4-tropic, GenBank accession number K03455). To produce a CCR5-tropic version of the clade B envelope protein (R5gp160/h), amino acids 275 to 361 from X4gp160/h (VRC3300) was replaced with the corresponding region from the BaL strain of HIV-1 (Genbank accession number M68893). The rAd5 vaccine consisted of four replication defective rAd5 vectors that utilized the same gene inserts as the DNA plasmid vaccine, with modifications as noted here. One vector encoded a Gag-Pol fusion product as previously described [48]. Nef was not included in the rAd5 vaccine. The remaining three vectors encoded secreted gp140 versions of the clade A, B and C Env described above. The clade B Env vector contained deletions of the V1 and V2 loop region in order to improve the stability and yield of the vector in the producer cell line. An control rAd5 vector contained no gene insert. The Env protein (dV2gp140) was a purified oligomeric gp140 derived from the CCR5 tropic strain SF162 and containing a 30 amino acid deletion in V2 loop region as has been previously been described [6, 39–41].
2.2 Immunogen preparation and vaccination
The DNA vaccine was formulated as equal parts of each of the six plasmids in phosphate-buffered saline (PBS) and was administered as a 500ug dose by intramuscular injection of 400 ul into one hind leg muscle using a needle-less biojector device. The rAd5 vectors were formulated in PBS in a 3:1:1:1 ratio of the Gag-Pol and three Env vectors respectively (i.e., half the formulation is the Gag-Pol vector and the other half contains the three Env vectors). The total dose per immunization was 1010 particle units delivered as 400 ul into one hind leg by needle and syringe. The SF162 protein was formulated in PBS (and in some cases with the indicated CpG ODN) and mixed with an equal volume of the oil emulsion MF59 adjuvant prior to immunization. The protein dose was 50 mcg per immunization delivered by needle and syringe in a volume of 400 ul and administered as 200 ul into each hind leg muscle. When GpG ODN were included, they were formulated with protein (not with DNA or rAd5) and the dose was 100 mcg per animal. ODN-7909 and ODN-2395, a class B and C ODN respectively, were purchased from Coley Pharmaceutical Group, Inc (Ottowa, Canada). Note that ODN-2006 has more recently been designated as ODN-7909 and in being studied in human trials [50]. Female Hartley guinea pigs were housed and handle in accordance with guidelines set by the Association for the Assessment and Accreditation of Laboratory Animal Care. Blood was collected at the specified time points and isolated serum was stored at −80ºC until use.
2.3 Antibody binding assay
HIV-1 gp140-specific binding antibodies were quantified by a lectin capture enzyme-linked immunosorbent assay (ELISA) as previously described [48, 51]. Briefly, 96-well Immulon2 2HB ELISA were coated with 100 ul/well of Galanthus Nivalis lectin (Sigma, St. Louis, Mo) (10 ug/ml in PBS) overnight at 4ºC and supernatant from 293T cells transfected with the BaL gp140 dCFI plasmid vaccine construct was added to each well for one hour at room temperature. After washing, serial dilution of guinea pig sera was added and anti-gp140 antibodies were detected with a biotin-labeled secondary goat anti-guinea pig IgG, and Streptavidin conjugated with horseradish peroxidase, followed by development with TMB (3, 5’, 5,5’-tetra-methylbenzidine) substrate. Plates were read on a Spectramax microplate spectrophotometer (Molecular Devices; Sunnyvale, CA) and end-point titer was calculated as the most dilute serum concentration that gave an optical density reading of > 0.2 after correction for background or pre-immune sera.
2.4 Virus neutralization assay
Single round of infection HIV-1 Env pseudoviruses were prepared by cotransfecting 293T cells with an Env expression plasmid containing a full gp160 env gene and an env-deficient HIV-1 backbone vector (pSG3ΔEnv) as previously described [52]. Virus-containing culture supernatants were harvested two days after transfection, centrifuged and filtered through 0.45-micron filter, and stored at −80°C. A panel of 12 clade B HIV-1 Env pseudoviruses has recently been characterized and recommended for use in assessing neutralization by HIV-1 immune sera [52, 53]. We used this panel of Env-pseudoviruses to assess the neutralization activity of the GP sera. In addition, several neutralization sensitive Env-pseudoviruses were used. These included isolates HxB2, SF162, BaL.01 and SS1196.1. The Env expression plasmid HxB2 was a gift from Dana Gabuzda (Dana Farber Cancer Institute) and SF162 was provided by Leonidas Stamatatos (Seattle Biomedical Research Institute). Env-pseudovirus SS1196.1 has been previously described [52]. Additionally, we isolated two functional Env clones from the DNA of peripheral blood mononuclear cells infected with the primary isolate HIV-1 BaL that was obtained from the NIH AIDS Research and Reference Reagent Program. The Env clones BaL.01 and BaL.26 differ by 16 amino acid positions in gp160 and have GenBank accession numbers DQ318210 and DQ318211 respectively. The BaL.01 pseudovirus is closer to the original BaL GenBank sequence and was used in this study.
Pseudovirus neutralization was measured as a function of Tat-induced luciferase reporter gene expression after a single round of infection in TZM-bl cells as previously described[52, 54]. TZM-bl cells were obtained from the NIH AIDS Research and Reference Reagent Program, as contributed by John Kappes and Xiaoyun Wu. These cells express CD4, CXCR4 and CCR5 and contain and integrated reporter gene for firefly luciferase under the control of an HIV-1 LTR. The level of viral infection was quantified by measurement of relative luciferase units (RLU) that are directly proportion to the amount of virus inputs. Briefly, 40 ul of virus was incubated for 30 minutes at 37ºC with serial dilutions of test serum samples (10 ul) in duplicate wells of a 96-well flat bottom culture plate. The final serum dilution was defined at the point of incubation with virus supernatant. 10,000 TZM-bl cells were then added to each well in a total volume of 20 ul and plates were incubated overnight at 37ºC in a 5% CO2 incubator. One set of eight wells received mock antibody followed by virus and cells (controls wells for virus entry) and a set of eight wells received cells with mock virus (to control for luciferase background). Viral input was set at a multiplicity of infection (moi) of approximately 0.1, which generally results in 100,000 to 400,00 RLU. After over night incubation, 150ul of fresh medium was added to each well and incubated for 24 hours at 37ºC in a 5% CO2 incubator. To determine RLU, cell culture medium was aspirated from wells followed by addition of 50 ul of cell lysis buffer (Promega, Madison, WI). 30 ul of cell lysate was transferred to wells of a black Optiplate (PerkinElmer) for measurement of luminescence using a Perkin-Elmer Victor-light luminometer that injects 50 ul of luciferase substrate reagent to each well just prior to reading RLU. To determine the serum inhibitory concentration that resulted in a 50% reduction in RLU, serum dilution neutralization curves were fit by non-linear regression using a 4-paremeter hill slope equation programmed into JMP statistical software (JMP 5.1, SAS Institute Inc., Cary, NC). The results are reported as the serum neutralization IC50, which is the reciprocal of the serum dilution producing 50% virus neutrarization.
Statistical analysis
Comparisons of end point ELISA titers or of IC50 neutralization titers at a specified time point were performed by Mann-Whitney or Wilcoxon signed-rank sum tests using the statistical package within GraphPad Prism (V4.03) software.
3. Results
3.1 Immunization protocol
This study was designed to test the effect of protein boosting after priming immunizations with either DNA or rAd5. In all cases, protein was formulated with the MF59 adjuvant. Since an additional objective was to evaluate if CpG ODNs could augment the antibody response produced by the MF59 formulation, we compared the MF59 adjuvant alone, to MF59 plus one of two ODNs (Fig. 1). Note that we were not using the ODN during the primary DNA or rAd5 immunizations, but rather only during the protein boosting phase. Guinea pigs were divided into 4 main groups as shown in Fig 1. 1) DNA prime + protein boost, 2) rAd5 prime + protein boost, 3) protein only immunization and 4) sham immunized controls. DNA groups were immunized with 500ug of plasmid DNA per guinea pig at week 0, 3 and 8. rAd5 immunization groups received a single dose of 1010 PU per animal at week 0. The twelve animals in both the DNA and rAd5 groups were further divided into three groups of 4 animals for protein boosting. One group received SF162 dV2gp140 Env protein formulated with MF59 + sham ODN. The other two group received Env protein formulated with MF59 and either ODN 7909 or ODN 2395 respectively. All DNA and rAd primed guinea pigs were boosted with protein at week 19 and 30. The comparator protein only group received SF162 gp140 protein formulated with MF59 (no ODN) at weeks 0, 3, 19 and 30. A sham immunization group received an empty rAd vector and sham protein (PBS) formulated with MF59.
Fig 1.
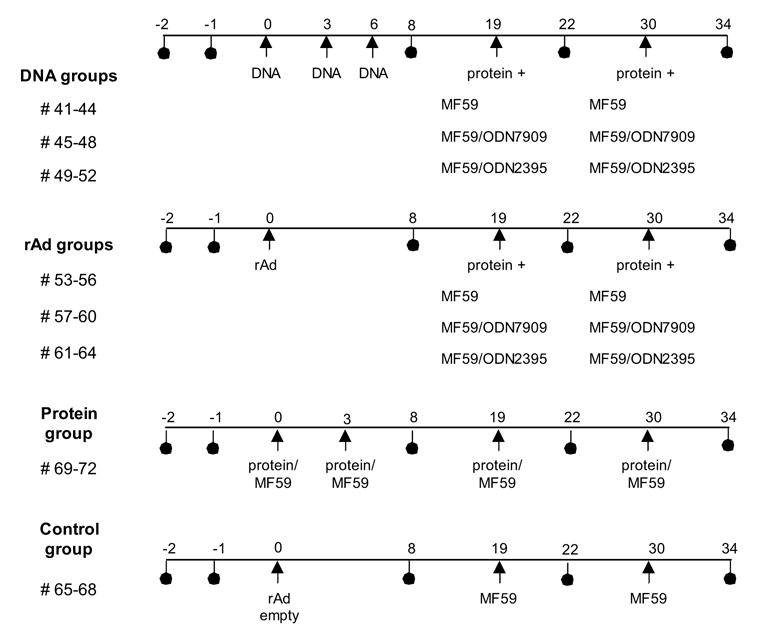
Schema of the immunization protocol. Arrows indicate immunizations and circles show serum collections. Thirty-two GPs were divided into eight groups of four animals. The first three groups (12 GPs) received DNA prime followed by protein boost and the second three groups received rAd prime, protein boost. The DNA groups received three i.m. inoculations of 500 ug of plasmid DNA and two 50 ug protein boosts. Protein was formulated with MF59, or with MF59 plus one of two GpG ODN as shown. The rAd groups each received one i.m. inoculation of 1010 PU of rAd and were boosted twice with 50 ug of protein. Protein was again formulated with MF59, or with MF59 plus the ODN. The protein only group received 50 ug i.m. protein formulated with MF59 at weeks 0, 3, 19 and 30. Control group GPs were immunized with an empty rAd vector at week 0 and were boosted with PBS/MF59 at weeks 19 and 30.
3.2 Comparison of DNA and rAd prime, protein boost
The plasmid DNA and rAd vectors used in this study encoded clade A, B and C Envs. The clade B Env is based on the strain HxB2, but includes 86 amino acids from the V3 region of the BaL strain. Thus, the chimeric clade B Env in the DNA and rAd5 is different from the SF162 based Env protein used to boost the antibody responses. We measured the anti-Env antibody titers using a clade B protein (HxB2/BaL gp140) because this matched the clade B Env insert in the DNA and rAd5. Since some antibodies are known to be highly strain specific, the ELISA titers measured for the protein only immunized animals (SF162 dV2gp140) might have been somewhat higher if they were measured using the homologous SF162 protein. However, we believe that reasonable comparisons could be made among groups by using the single HxB2/BaL gp140 protein for ELISA testing. Also of note, since we found that the CpG ODN did not alter the antibody response when added to MF59 (section 3.3 below), we combined all 12 GPs in the rAd5 + protein, and the DNA + protein groups, for the evaluations done here. We observed that three immunizations of plasmid DNA, and one immunization of rAd, each generated measurable serum ELISA titers (Fig 2A), though the response was more consistent in the rAd group. In the DNA immunized animals, a single protein boost increased the reciprocal geometric mean titer (GMT) from 12,000 to 386,000 (32-fold incease; p = 0.0005). In rAd immunized animals, the GMT increased from 23,000 to 1,546,000 after the protein boost (67-fold increase; p = 0.0001). In contrast, two protein immunizations (weeks 0, 3) resulted in a GMT of 6,400 that was significantly lower than a single protein immunization after DNA prime (p = 0.008) or rAd5 prime (p = 0.004). Thus, both DNA and rAd effectively primed for the protein boost immunization and the resulting GMTs were approximately 100-fold higher than was observed after two protein immunizations in the absence of DNA or rAd. In the protein only group, a third protein immunization at week 19 brought the GMT up to 102,000, which was still several fold lower than observed in the DNA and rAd primed animals. A comparison of the DNA + protein and rAd + protein groups shows GMTs of 386,000 and 1,546,000 respectively (p = 0.053). Thus, there was a trend toward a higher GMT titer in the rAd + protein animals. All animals were given an additional protein immunization at week 30, but this do not provide a significant increase in the GMT (Fig 2B). All P values described above were derived from non-parametric Mann Whitney tests of the IC50 values.
Fig 2.
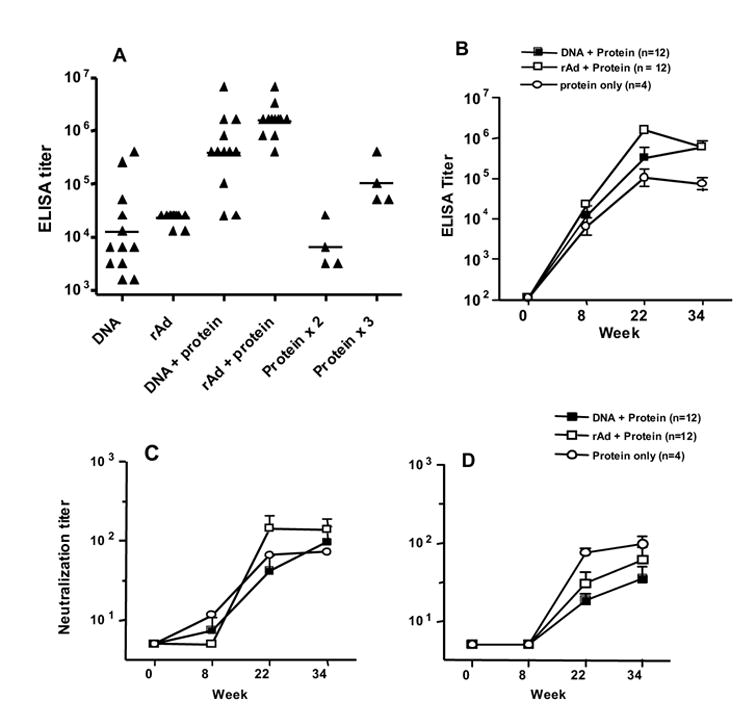
Comparison of DNA and rAd prime. (A) Anti-Env ELISA titers for the 12 GPs that received 3 DNA immunizations (DNA) and the 12 GPs that received one rAd5 immunization (rAd); the time point shown is week 8. ELISA titers are also shown from sera sampled at week 22, two weeks after the first protein boost immunization (DNA + protein, DNA + rAd). The two columns furthest to the right show data from the four protein only immunized GPs; time points are week 8 (after protein x 2) and week 22 (after protein x 3). The line within each group of scatter points indicates the GMT. (B) Longitudinal ELISA titers for the same animals described above. The immunization scheme is shown above in Fig. 1. (C) Neutralization IC50 values for viral isolate SS1196. (D) Neutralization IC50 values for viral isolate BaL.01. All line graphs show the geometric mean value and SEM for each time point.
Neutralizing antibody responses at several time points were first assessed against two neutralization sensitive HIV-1 strains: SS1196 (Fig 2C) and BaL.01 (Fig. 2D) . Serum neutralizing activity was detected after DNA or rAd prime followed by a single protein boost (Fig 2C and 2D, week 22) or after three protein immunizations (also week 22). Overall, the neutralization IC50 titers against BaL.01 and SS1196 were in the low to moderate range. While there was some small increase in titer with an additional protein boost (week 34) this increase was not statistically significant. Interestingly, the protein only group of animals was more potently neutralizing against BaL.01 than the DNA or rAd groups, but this was not seen for SS1196. The reason for this difference is not clear. It is known that current Env immunogens, including those used here, do not elicit neutralizing responses against the majority of primary HIV-1 strains. To more formally assess the breadth of neutralization by these sera, they were tested against two additional neutralization sensitive viruses (HxB2 and SF162) and against a panel of 12 heterologous clade B reference viruses that that have recently been recommended for this purpose [52, 53]. There was strong neutralization of SF162, which is homologous to the protein boost, especially in the animals that received three or four immunizations with the SF162 dVgp140 protein. This can be seen in Table 1 where the protein only immunized animals have particularly high neutralization titers against the homologous SF162 virus that is stronger than that seen with the DNA or rAd5 immunized animals. This is likely due to a subset of highly strain specific antibodies elicited by the SF162 dV2gp140 protein that are reactive with both SF162 and the highly sensitive laboratory adapted HxB2 strain. But this neutralization does not extend to the other primary isolates tested. Among the 12 reference strains tested, we observed some neutralization of two viruses (6535.3 and SC422.8). There was little or no neutralization of the remaining 10 viruses. Table 1 shows data for only four of the reference viruses. There was no detectable neutralization against the remaining eight viruses and these data are not shown.
Table 1.
Neutralizing antibody response against an extended panel of clade B panel viruses
| HXB2 | SF162 | 6535.03 | SC422.8 | QH0692.42 | WITO.33 | MuLV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Immunogen | Animals | wk 22 | wk 34 | wk 22 | wk 34 | wk 22 | wk 34 | wk 22 | wk 34 | wk 22 | wk 34 | wk 22 | wk 34 | wk 22 | wk 34 |
| DNA + Protein (MF59) | 41 | - | 587 | 12 | 5626 | - | - | - | 61 | - | - | - | - | - | - |
| 42 | - | 33 | - | 469 | - | - | - | 20 | - | 13 | - | - | - | - | |
| 43 | - | - | 39 | 2096 | - | 82 | - | - | - | - | - | - | - | - | |
| 44 | - | 301 | 19 | 7253 | - | 140 | - | - | - | - | - | - | - | - | |
| DNA + Protein (MF59/ODN7909) | 45 | - | 48 | 510 | 1182 | - | 20 | - | 65 | - | - | - | - | - | - |
| 46 | - | - | 17 | 2231 | - | 105 | - | - | - | - | - | 18 | - | - | |
| 47 | - | - | - | 4189 | - | - | - | 44 | - | - | - | 19 | - | - | |
| 48 | - | 2272 | - | 2643 | - | 79 | - | 42 | - | - | - | - | - | - | |
| DNA + Protein (MF59/ODN2395) | 49 | - | nd | 365 | nd | - | nd | - | nd | - | nd | - | nd | - | nd |
| 50 | - | - | 48 | 3300 | - | 95 | - | 25 | - | - | - | - | - | - | |
| 51 | - | 155 | 50 | 3135 | - | - | - | - | - | - | - | - | - | - | |
| 52 | - | 45 | 22 | 661 | - | - | - | - | - | - | - | - | - | - | |
| rAd + Protein (MF59) | 53 | - | 120 | 164 | 1260 | - | - | - | - | - | - | - | - | - | - |
| 54 | - | 19 | 41 | 151 | - | - | - | - | - | - | - | - | - | - | |
| 55 | - | - | 129 | 249 | - | - | - | - | - | - | - | - | - | - | |
| 56 | - | - | 592 | 1009 | - | - | - | - | - | - | - | - | - | - | |
| rAd + Protein (MF59/ODN7909) | 57 | - | 323 | 86 | 1065 | - | 85 | - | - | - | - | - | - | - | - |
| 58 | 18 | 59 | 179 | 750 | - | 24 | - | - | - | - | - | - | - | - | |
| 59 | - | 22 | 37 | 260 | - | - | - | - | - | - | - | - | - | - | |
| 60 | - | 164 | 5593 | 3892 | 570 | 387 | - | - | - | - | - | - | - | - | |
| rAd + Protein (MF59/ODN2395) | 61 | - | 170 | 144 | 1098 | - | - | - | - | - | - | - | - | - | - |
| 62 | 33 | 62 | 400 | 2145 | - | 198 | - | - | - | - | - | - | - | - | |
| 63 | - | 33 | 26 | 321 | - | 18 | - | - | - | - | - | - | - | - | |
| 64 | - | 39 | - | 218 | - | - | - | - | - | - | - | - | - | - | |
| Protein only (MF59) | 69 | 274 | 610 | 7181 | 9344 | - | 29 | - | - | - | - | - | - | - | - |
| 70 | 20 | 29 | 1176 | 1675 | - | - | - | - | - | - | - | - | - | - | |
| 71 | 392 | 450 | 9473 | 9855 | 76 | 58 | - | - | - | - | - | - | - | - | |
| 72 | 1689 | 2598 | 5856 | 8018 | 122 | 267 | - | - | - | - | - | - | - | - | |
| rAd empty vector | 65 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 66 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 67 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| 68 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
The vaccine modalities are shown in the left column. The adjuvant, shown in parenthesis, was used with protein immunizations only. Value indicate the reciprocal serum dilution that resulted in 50% neutralization of each virus. Time points shown are week 22 and week 34. A dash (-) indicates that 50% neutralization was not resorded at the lowest serum dilution tested (1:10). nd indicates not determined.
3.2 Comparison of protein formulated with ODN protein
We further evaluated if GpG ODN could augment the antibody response elicited by SF162 protein formulated in MF59. To asses this, we added CpG ODN to MF59 during the boost phase of the immunization regimen. The 12 GPs in DNA/protein and rAd/protein immunization groups were subdivided into three groups of 4 animals as shown if Fig 1. GPs received SF162 gp140 with MF59, or with MF59 plus ODN7909 or ODN2395. Fig. 3A shows that the there were no differences in the ELISA titers at any time point tested for the DNA/protein groups (left panel) or the rAd/protein groups (right panel). We also assessed the neutralizing antibody response against viruses SS1196 (Fig 3B) and BaL.01 (Fig 3C). Again, there were no statistically significant differences in the IC50 neutralization titers at any time point tested. Thus, although these ODN have been found to be effective adjuvants during primary immunization, we found no evidence that a class B of C ODN (used at a dose of 100 mcg per animal) could augment the antibody response generated by the protein/MF59 formulation in the setting of an immune response primed by DNA or rAd5.
Fig 3.
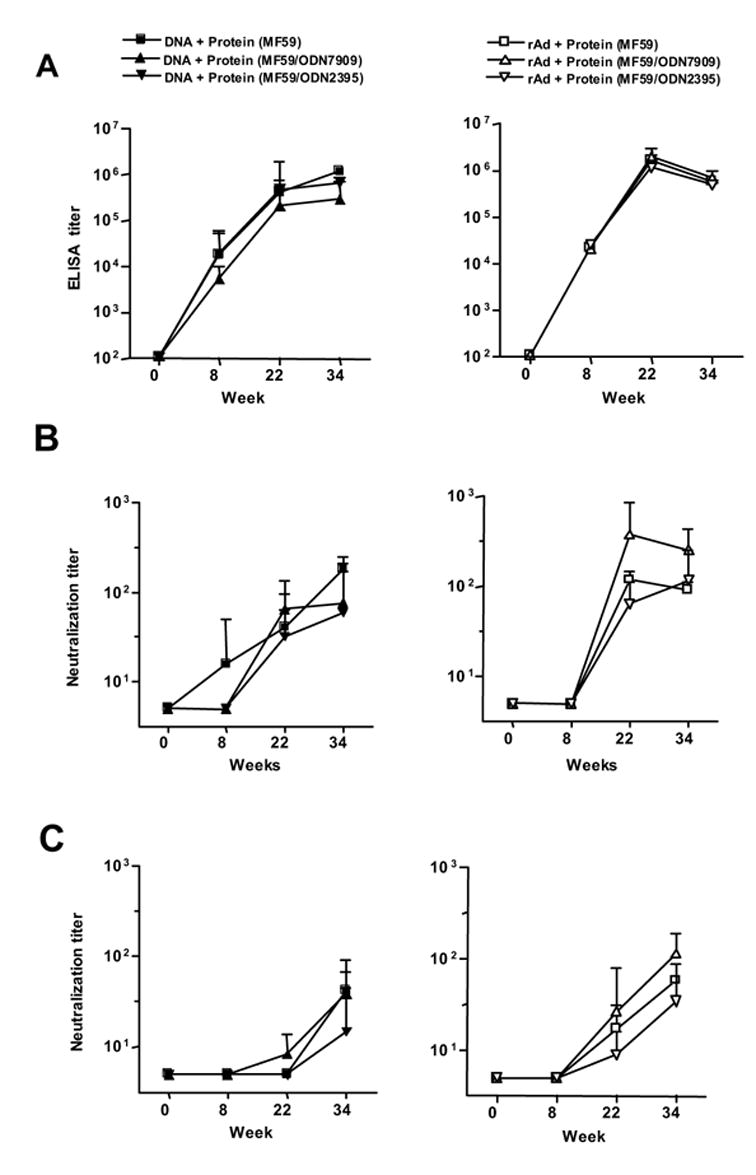
Comparison of MF59 and GpG ODN adjuvants. The symbols at the top of the left and right columns describe the animal groups. (A) Anti-Env ELISA titers are shown for DNA (left panel) and rAd (right panel) immunized animals. Note that the graphs for MF59 used alone or combined with an ODN are overlapping. Also shown are Neutralization IC50 titers for viral isolate SS1196 (B) and viral isolate BaL.01 (C).. The line graphs show the geometric mean value and SEM for each time point; there were four animals in each group.
6. Discussion
It this study, we focused on several HIV-1 candidate vaccines that are being tested in ongoing phase I/II clinical trails; these included a multigene plasmid DNA, a multigene rAd5 vaccine, and a soluble clade B trimeric dV2gp140. While protein immunization can induce high antibody levels, it does not elicit the CD8 T-cell responses that are thought to be important for controlling HIV-1 infection. Since DNA and rAd5 vectors elicit robust cellular immune responses, including CD4 T-cell response to the Env antigens, we hypothesized that they would prime for a protein boost that could further augment the antibody levels induced by these candidate vaccines. We observed that three DNA immunizations, or a single rAd5 immunization, resulted in moderate levels of anti-Env antibodies that were boosted by approximately 50-fold upon protein boosting. The resulting antibody response was high enough to neutralize several strains of HIV-1, though as expected these neutralizing antibodies were not broadly reactive.
The dV2gp140 SF162 Env protein used here has been previously studied in NHP. It has been shown to boost the antibody response after immunization with DNA plasmids encoding the same SF162 Env. This resulted in a potent neutralizing response against the homologous SF162 virus and partial protection against a SHIV SF162 challenge [6, 43]. However, the reactivity of neutralizing antibodies was limited and additional SHIV challenge studies showed that protection did not extend to a heterologous SHIV89.6P challenge [42]. In the current study, we primed with DNA or rAd5 that encoded for a clade A, B and C Env. The clade B Env was a derived from the HxB2 sequence that was modified to include 86 amino acids from the V3 region of the BaL strain. Thus, the priming Env immunizations were antigenically broader than the boost and were not directly homologous to the SF162 protein. We therefore performed an extensive analysis of the breadth of the neutralizing antibody response at a peak time point after the protein boost, and again after an additional protein boost. For this purpose, we used several well characterized neutralization sensitive viruses and a recently recommended panel of clade B reference isolates. As expected, moderately high neutralization titers were observed against the more sensitive viruses: BaL, SS1196, SF162 and HxB2. Among the new panel of 12 reference clade B strains, there were moderate level responses only against virus 6535 (Table 1). This result is not surprising, as several studies have demonstrated that these current Env immunogens do not yet elicit broadly reactive neutralizing antibodies [6, 13, 35].
Interestingly, the serum neutralizing activity was somewhat higher at week 34, after the second protein boost, compared to week 22 (after the first protein boost). This can be seen in Table 1 for viruses HxB2, SF162, 6535 and SC422. Thus, despite the fact that the second protein boost did not substantially increase the ELISA titer measured against BaL gp120, the virus neutralizing activity was improved. This is not surprising, as previous studies have failed to find a correlation between gp120 binding levels and virus neutralization[55, 56]. The fraction of total anti-Env antibodies that are neutralizing may be small, and boosting this fraction may have minimal effect on the total anti-gp120 ELISA titer. Also of note, the protein only immunized animals had higher neutralization against SF162 and HxB2 than the animals primed with DNA or rAd5 (Table 1). This is likely due to a set of highly type specific antibodies elicited by the SF162 dV2gp140 protein, as these sera from protein only immunization do not neutralize most heterologous viruses. Thus, using current Env immunogens, neither homologous protein alone, nor DNA or rAd5 prime with heterologous protein boost, was able to induce broadly reactive neutralizing antibodies.
This study did not detect an effect of GpG ODN on the antibody response, but it is important to note that we did not design these experiments to study ODN during the primary set of immunizations. Synthetic ODN can potently augment Th1 driven immune responses in mice [45] and have recently been shown to have similar properties in NHPs and humans [57] [50]. Much less is known about their effect in GPs, although CpG ODN have been reported to augment the response in GPs to mycobacterial antigens [58], and the anti-HIV-1 Env response after immunization with virus like particles [59]. Two aspects of our study design may have minimized the effect of the ODN. First, we only studied the effect of ODN on protein used to boost an existing immune response generated with DNA or rAd5 prime. Since both these modalities generated an antibody response, there may have been less potential for the ODN to augment the secondary immune response. Secondly, we used a well characterized adjuvant (MF59) in all protein formulations. Thus, we can only conclude that there was no effect of ODN above that produced by the MF59 alone. It is also possible that a higher dose of the ODN than the 100 mcg used here would have produced a more pronounced effect.
In summary, a successful HIV-1 vaccine will likely need to generate both cellular immunity and neutralizing antibodies. While intense research continues to focus on the design of more optimal Env antigens , it is also important to understand how such Env immunogens might be delivered as part of an overall HIV vaccine strategy aimed at generating both cellular immunity and neutralizing antibodies. Gene-based vectors such as plasmid DNA and rAd5 can effectively express Env antigens and induce both T-cell and antibody responses. In this study, we show that both DNA and rAd5 can each efficiently prime for protein boosting. Thus, immunization with DNA or rAd5, plus protein boosting, is a useful platform to study the immune response to novel Env immunogens and may allow the induction of both CD8 T-cell and neutralizing antibody responses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat Med. 2004;10(8):806–10. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- 2.Burton DR, Desrosiers RC, Doms RW, Koff WC, Kwong PD, Moore JP, et al. HIV vaccine design and the neutralizing antibody problem. Nat Immunol. 2004;5(3):233–6. doi: 10.1038/ni0304-233. [DOI] [PubMed] [Google Scholar]
- 3.Mascola JR. Defining the protective antibody response for HIV-1. Curr Mol Med. 2003;3(3):209–16. doi: 10.2174/1566524033479799. [DOI] [PubMed] [Google Scholar]
- 4.Cho MW, Kim YB, Lee MK, Gupta KC, Ross W, Plishka R, et al. Polyvalent Envelope Glycoprotein Vaccine Elicits a Broader Neutralizing Antibody Response but Is Unable To Provide Sterilizing Protection against Heterologous Simian/Human Immunodeficiency Virus Infection in Pigtailed Macaques. J Virol. 2001;75(5):2224–34. doi: 10.1128/JVI.75.5.2224-2234.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mascola JR, Snyder SW, Weislow OS, Belay SM, Belshe RB, Schwartz DH, et al. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis. 1996;173(2):340–8. doi: 10.1093/infdis/173.2.340. [DOI] [PubMed] [Google Scholar]
- 6.Barnett SW, Lu S, Srivastava I, Cherpelis S, Gettie A, Blanchard J, et al. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J Virol. 2001;75(12):5526–40. doi: 10.1128/JVI.75.12.5526-5540.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polacino PS, Stallard V, Klaniecki JE, Pennathur S, Montefiori DC, Langlois AJ, et al. Role of immune responses against the envelope and the core antigens of simian immunodeficiency virus SIVmne in protection against homologous cloned and uncloned virus challenge in Macaques. J Virol. 1999;73(10):8201–15. doi: 10.1128/jvi.73.10.8201-8215.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.VanCott TC, Mascola JR, Kaminski RW, Kalyanaraman V, Hallberg PL, Burnett PR, et al. Antibodies with specificity to native gp120 and neutralization activity against primary human immunodeficiency virus type 1 isolates elicited by immunization with oligomeric gp160. J Virol. 1997;71(6):4319–30. doi: 10.1128/jvi.71.6.4319-4330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, et al. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci U S A. 2005;102(42):15190–4. doi: 10.1073/pnas.0507484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amara RR, Villinger F, Altman JD, Lydy SL, O'Neil SP, Staprans SI, et al. Control of a Mucosal Challenge and Prevention of AIDS by a Multiprotein DNA/MVA Vaccine. Science. 2001;292(5514):69–74. doi: 10.1126/science.1058915. [DOI] [PubMed] [Google Scholar]
- 11.Shiver JW, Fu TM, Chen L, Casimiro DR, Davies ME, Evans RK, et al. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature. 2002;415(6869):331–5. doi: 10.1038/415331a. [DOI] [PubMed] [Google Scholar]
- 12.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME, et al. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77(11):6305–13. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seaman MS, Xu L, Beaudry K, Martin KL, Beddall MH, Miura A, et al. Multiclade human immunodeficiency virus type 1 envelope immunogens elicit broad cellular and humoral immunity in rhesus monkeys. J Virol. 2005;79(5):2956–63. doi: 10.1128/JVI.79.5.2956-2963.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouch DH. Rational design of gene-based vaccines. J Pathol. 2006;208(2):283–9. doi: 10.1002/path.1874. [DOI] [PubMed] [Google Scholar]
- 15.Doria-Rose NA, Ohlen C, Polacino P, Pierce CC, Hensel MT, Kuller L, et al. Multigene DNA priming-boosting vaccines protect macaques from acute CD4+-T-cell depletion after simian-human immunodeficiency virus SHIV89.6P mucosal challenge. J Virol. 2003;77(21):11563–77. doi: 10.1128/JVI.77.21.11563-11577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacGregor RR, Ginsberg R, Ugen KE, Baine Y, Kang CU, Tu XM, et al. T-cell responses induced in normal volunteers immunized with a DNA-based vaccine containing HIV-1 env and rev. Aids. 2002;16(16):2137–43. doi: 10.1097/00002030-200211080-00005. [DOI] [PubMed] [Google Scholar]
- 17.Cebere I, Dorrell L, McShane H, Simmons A, McCormack S, Schmidt C, et al. Phase I clinical trial safety of DNA- and modified virus Ankara-vectored human immunodeficiency virus type 1 (HIV-1) vaccines administered alone and in a prime-boost regime to healthy HIV-1-uninfected volunteers. Vaccine. 2006;24(4):417–25. doi: 10.1016/j.vaccine.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 18.Catanzaro AT, Koup RA, Roederer M, Bailer R, Enama ME, Moodie Z, et al. Phase I Safety and Immunogenicity Evaluation of a Multiclade HIV-1 Recombinant Adenoviral Vector Vaccine in Uninfected Adults. Journal of Infectious Diseases. 2006 In Press. [Google Scholar]
- 19.Graham BS, Koup RA, Roederer M, Bailer R, Enama ME, Moodie Z, et al. A Multiclade HIV-1 DNA Vaccine Elicits Humoral and Sustained Cellular Immune Responses in Humans in a Randomized Controlled Phase I Clinical Trial. Journal of Infectious Diseases. 2006 In Press. [Google Scholar]
- 20.Barnett SW, Rajasekar S, Legg H, Doe B, Fuller DH, Haynes JR, et al. Vaccination with HIV-1 gp120 DNA induces immune responses that are boosted by a recombinant gp120 protein subunit. Vaccine. 1997;15(8):869–73. doi: 10.1016/s0264-410x(96)00264-2. [DOI] [PubMed] [Google Scholar]
- 21.Letvin NL, Montefiori DC, Yasutomi Y, Perry HC, Davies ME, Lekutis C, et al. Potent, protective anti-HIV immune responses generated by bimodal HIV envelope DNA plus protein vaccination. Proc Natl Acad Sci U S A. 1997;94(17):9378–83. doi: 10.1073/pnas.94.17.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu SL, Abrams K, Barber GN, Moran P, Zarling JM, Langlois AJ, et al. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992;255(5043):456–9. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- 23.Montefiori DC, Graham BS, Kliks S, Wright PF. Serum antibodies to HIV-1 in recombinant vaccinia virus recipients boosted with purified recombinant gp160. NIAID AIDS Vaccine Clinical Trials Network. J Clin Immunol. 1992;12(6):429–39. doi: 10.1007/BF00918855. [DOI] [PubMed] [Google Scholar]
- 24.Polacino P, Stallard V, Montefiori DC, Brown CR, Richardson BA, Morton WR, et al. Protection of macaques against intrarectal infection by a combination immunization regimen with recombinant simian immunodeficiency virus SIVmne gp160 vaccines. J Virol. 1999;73(4):3134–46. doi: 10.1128/jvi.73.4.3134-3146.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leung L, Srivastava IK, Kan E, Legg H, Sun Y, Greer C, et al. Immunogenicity of HIV-1 Env and Gag in baboons using a DNA prime/protein boost regimen. Aids. 2004;18(7):991–1001. doi: 10.1097/00002030-200404300-00006. [DOI] [PubMed] [Google Scholar]
- 26.Patterson LJ, Malkevitch N, Venzon D, Pinczewski J, Gomez-Roman VR, Wang L, et al. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J Virol. 2004;78(5):2212–21. doi: 10.1128/JVI.78.5.2212-2221.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S, Arthos J, Lawrence JM, Van Ryk D, Mboudjeka I, Shen S, et al. Enhanced immunogenicity of gp120 protein when combined with recombinant DNA priming to generate antibodies that neutralize the JR-FL primary isolate of human immunodeficiency virus type 1. J Virol. 2005;79(12):7933–7. doi: 10.1128/JVI.79.12.7933-7937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cristillo AD, Wang S, Caskey MS, Unangst T, Hocker L, He L, et al. Preclinical evaluation of cellular immune responses elicited by a polyvalent DNA prime/protein boost HIV-1 vaccine. Virology. 2006;346(1):151–68. doi: 10.1016/j.virol.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Pal R, Mascola JR, Chou TH, Mboudjeka I, Shen S, et al. Polyvalent HIV-1 Env vaccine formulations delivered by the DNA priming plus protein boosting approach are effective in generating neutralizing antibodies against primary human immunodeficiency virus type 1 isolates from subtypes A, B, C, D and E. Virology. 2006;350(1):34–47. doi: 10.1016/j.virol.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 30.Mossman SP, Pierce CC, Watson AJ, Robertson MN, Montefiori DC, Kuller L, et al. Protective immunity to SIV challenge elicited by vaccination of macaques with multigenic DNA vaccines producing virus-like particles. AIDS Res Hum Retroviruses. 2004;20(4):425–34. doi: 10.1089/088922204323048177. [DOI] [PubMed] [Google Scholar]
- 31.Santra S, Seaman MS, Xu L, Barouch DH, Lord CI, Lifton MA, et al. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J Virol. 2005;79(10):6516–22. doi: 10.1128/JVI.79.10.6516-6522.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Letvin NL, Mascola JR, Sun Y, Gorgone DA, Buzby AP, Xu L, et al. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science. 2006;312(5779):1530–3. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, et al. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag. J Virol. 2005;79(24):15547–55. doi: 10.1128/JVI.79.24.15547-15555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suh YS, Park KS, Sauermann U, Franz M, Norley S, Wilfingseder D, et al. Reduction of viral loads by multigenic DNA priming and adenovirus boosting in the SIVmac-macaque model. Vaccine. 2006;24(11):1811–20. doi: 10.1016/j.vaccine.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Mascola JR, Sambor A, Beaudry K, Santra S, Welcher B, Louder MK, et al. Neutralizing antibodies elicited by immunization of monkeys with DNA plasmids and recombinant adenoviral vectors expressing human immunodeficiency virus type 1 proteins. J Virol. 2005;79(2):771–9. doi: 10.1128/JVI.79.2.771-779.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDermott AB, O'Connor DH, Fuenger S, Piaskowski S, Martin S, Loffredo J, et al. Cytotoxic T-lymphocyte escape does not always explain the transient control of simian immunodeficiency virus SIVmac239 viremia in adenovirus-boosted and DNA-primed Mamu-A*01-positive rhesus macaques. J Virol. 2005;79(24):15556–66. doi: 10.1128/JVI.79.24.15556-15566.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J, et al. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol. 2006;80(12):5875–85. doi: 10.1128/JVI.00171-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava IK, Stamatatos L, Legg H, Kan E, Fong A, Coates SR, et al. Purification and characterization of oligomeric envelope glycoprotein from a primary R5 subtype B human immunodeficiency virus. J Virol. 2002;76(6):2835–47. doi: 10.1128/JVI.76.6.2835-2847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stamatatos L, Cheng-Mayer C. An envelope modification that renders a primary, neutralization- resistant clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J Virol. 1998;72(10):7840–5. doi: 10.1128/jvi.72.10.7840-7845.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srivastava IK, Stamatatos L, Kan E, Vajdy M, Lian Y, Hilt S, et al. Purification, characterization, and immunogenicity of a soluble trimeric envelope protein containing a partial deletion of the V2 loop derived from SF162, an R5-tropic human immunodeficiency virus type 1 isolate. J Virol. 2003;77(20):11244–59. doi: 10.1128/JVI.77.20.11244-11259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava IK, VanDorsten K, Vojtech L, Barnett SW, Stamatatos L. Changes in the immunogenic properties of soluble gp140 human immunodeficiency virus envelope constructs upon partial deletion of the second hypervariable region. J Virol. 2003;77(4):2310–20. doi: 10.1128/JVI.77.4.2310-2320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu R, Srivastava IK, Kuller L, Zarkikh I, Kraft Z, Fagrouch Z, et al. Immunization with HIV-1 SF162-derived Envelope gp140 proteins does not protect macaques from heterologous simian-human immunodeficiency virus SHIV89.6P infection. Virology. 2006;349(2):276–89. doi: 10.1016/j.virol.2006.01.043. [DOI] [PubMed] [Google Scholar]
- 43.Buckner C, Gines LG, Saunders CJ, Vojtech L, Srivastava I, Gettie A, et al. Priming B cell-mediated anti-HIV envelope responses by vaccination allows for the long-term control of infection in macaques exposed to a R5-tropic SHIV. Virology. 2004;320(1):167–80. doi: 10.1016/j.virol.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 45.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 46.Jurk M, Schulte B, Kritzler A, Noll B, Uhlmann E, Wader T, et al. C-Class CpG ODN: sequence requirements and characterization of immunostimulatory activities on mRNA level. Immunobiology. 2004;209(1–2):141–54. doi: 10.1016/j.imbio.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Barouch DH, Yang ZY, Kong WP, Korioth-Schmitz B, Sumida SM, Truitt DM, et al. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J Virol. 2005;79(14):8828–34. doi: 10.1128/JVI.79.14.8828-8834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong WP, Huang Y, Yang ZY, Chakrabarti BK, Moodie Z, Nabel GJ. Immunogenicity of multiple gene and clade human immunodeficiency virus type 1 DNA vaccines. J Virol. 2003;77(23):12764–72. doi: 10.1128/JVI.77.23.12764-12772.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chakrabarti BK, Kong WP, Wu BY, Yang ZY, Friborg J, Ling X, et al. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J Virol. 2002;76(11):5357–68. doi: 10.1128/JVI.76.11.5357-5368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Krieg AM. CpG motifs: the active ingredient in bacterial extracts? Nat Med. 2003;9(7):831–5. doi: 10.1038/nm0703-831. [DOI] [PubMed] [Google Scholar]
- 51.Chakrabarti BK, Ling X, Yang ZY, Montefiori DC, Panet A, Kong WP, et al. Expanded breadth of virus neutralization after immunization with a multiclade envelope HIV vaccine candidate. Vaccine. 2005;23(26):3434–45. doi: 10.1016/j.vaccine.2005.01.099. [DOI] [PubMed] [Google Scholar]
- 52.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, et al. Human Immunodeficiency Virus Type 1 env Clones from Acute and Early Subtype B Infections for Standardized Assessments of Vaccine-Elicited Neutralizing Antibodies. J Virol. 2005;79(16):10108–25. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mascola JR, D'Souza P, Gilbert P, Hahn BH, Haigwood NL, Morris L, et al. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J Virol. 2005;79(16):10103–7. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Svehla K, Mathy NL, Voss G, Mascola JR, Wyatt R. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J Virol. 2006;80(3):1414–26. doi: 10.1128/JVI.80.3.1414-1426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fouts TR, Trkola A, Fung MS, Moore JP. Interactions of polyclonal and monoclonal anti-glycoprotein 120 antibodies with oligomeric glycoprotein 120-glycoprotein 41 complexes of a primary HIV type 1 isolate: relationship to neutralization. AIDS Res Hum Retroviruses. 1998;14(7):591–7. doi: 10.1089/aid.1998.14.591. [DOI] [PubMed] [Google Scholar]
- 56.Parren PW, Wang M, Trkola A, Binley JM, Purtscher M, Katinger H, et al. Antibody neutralization-resistant primary isolates of human immunodeficiency virus type 1. J Virol. 1998;72(12):10270–4. doi: 10.1128/jvi.72.12.10270-10274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, et al. Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med. 2006;203(5):1249–58. doi: 10.1084/jem.20052433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hogarth PJ, Jahans KJ, Hecker R, Hewinson RG, Chambers MA. Evaluation of adjuvants for protein vaccines against tuberculosis in guinea pigs. Vaccine. 2003;21(9–10):977–82. doi: 10.1016/s0264-410x(02)00548-0. [DOI] [PubMed] [Google Scholar]
- 59.Hammonds J, Chen X, Fouts T, DeVico A, Montefiori D, Spearman P. Induction of neutralizing antibodies against human immunodeficiency virus type 1 primary isolates by Gag-Env pseudovirion immunization. J Virol. 2005;79(23):14804–14. doi: 10.1128/JVI.79.23.14804-14814.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


