Abstract
The mitochondrial phosphate carrier SLC25A3 transports inorganic phosphate into the mitochondrial matrix, which is essential for the aerobic synthesis of adenosine triphosphate (ATP). We identified a homozygous mutation—c.215G→A (p.Gly72Glu)—in the alternatively spliced exon 3A of this enzyme in two siblings with lactic acidosis, hypertrophic cardiomyopathy, and muscular hypotonia who died within the 1st year of life. Functional investigation of intact mitochondria showed a deficiency of ATP synthesis in muscle but not in fibroblasts, which correlated with the tissue-specific expression of exon 3A in muscle versus exon 3B in fibroblasts. The enzyme defect was confirmed by complementation analysis in yeast. This is the first report of patients with mitochondrial phosphate–carrier deficiency.
Defects in oxidative phosphorylation are a frequent cause of a disturbed mitochondrial energy metabolism and occur with a prevalence of at least 1 in 7,600.1 The clinical spectrum of this group of diseases is heterogeneous, with variable onset and course ranging from neonatal onset with early death to adult forms with milder clinical affection. Multiple organ involvement is frequently observed, but tissue-specific variants are also known. The broad clinical spectrum is mirrored at the molecular level by possible defects of >100 different proteins and genes of the nuclear and mitochondrial genome, complicating the diagnosis of these diseases at the molecular level.
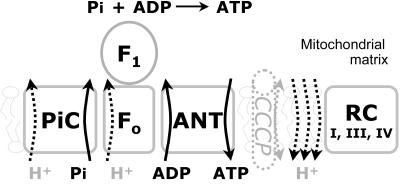
The respiratory-chain enzymes I, III, and IV create a proton gradient across the inner mitochondrial membrane during their oxidative reaction. This proton gradient is used for the production of adenosine triphosphate (ATP) and various transport processes (fig. 1). ATP is synthesized on the matrix side of the inner mitochondrial membrane from adenosine diphosphate (ADP) and inorganic phosphate by F1F0-ATP synthase. Import of inorganic phosphate into the mitochondrial matrix is an energy-dependent, oriented process that is catalyzed by the mitochondrial phosphate–carrier protein (PiC) termed “SLC25A3,” a member of the mitochondrial-carrier family. Mitochondrial carriers facilitate the transport of a variety of substances across the inner mitochondrial membrane. They are characterized by a common structure that includes six transmembrane helices, and they share conserved motifs in their primary sequence.2
Figure 1. .
Functional elements of mitochondrial ATP synthesis. Inorganic phosphate (Pi) is transported into the mitochondrial matrix by the mitochondrial PiC. F1F0-ATP synthase forms ATP from Pi and ADP. ATP is exchanged across the inner mitochondrial membrane with ADP by the ANT. The whole reaction is driven by a proton gradient, which is maintained mainly by the respiratory-chain (RC) complexes I, III, and IV. CCCP is an artificial proton pore that destroys the proton gradient across the mitochondrial inner membrane. In the presence of CCCP, the RC is maximally stimulated, but no ATP is formed.
Mitochondrial PiC is an essential part of the mitochondrial energy–producing machinery. Evidence comes from studies with yeast, a facultative anaerobe organism with dispensable oxidative phosphorylation on fermentable carbon sources (e.g., glucose). Absence of both isoforms of mitochondrial PiC, encoded by the MIR1 and PIC2 genes, renders cells viable on glucose but unable to grow on nonfermentable carbon sources.3 In mammals, mitochondrial PiC is ubiquitously expressed but is found in two distinct isoforms, termed “PiC-A” and “PiC-B.” These two isoforms originate from alternatively spliced transcripts that differ in a mutually exclusive exon; exon 3A has tissue-specific expression in heart and muscle, whereas exon 3B is the predominant isoform in other tissues.3,4 The alternatively spliced exons code for 42 and 41 aa in isoforms A and B, respectively, and are >70% identical. In vitro, the two isoforms display different substrate affinities and transport rates.5
Here, we describe two patients from a single family who had a cardiac and muscular disease and a tissue-specific defect in the mitochondrial PiC. Patient 1, a girl, the second child of nonconsanguineous Turkish parents, was born after an uneventful pregnancy in the 38th wk of gestation, with a birth weight of 2,600 g (10th–50th percentile), length of 47 cm (10th–50th percentile), head circumference of 33 cm (10th–50th percentile), and Apgar scores of 9, 10, and 10 (at ages 1, 5, and 10 min, respectively). At age 12 h, the child presented with cyanosis and muscular hypotonia that necessitated intensive-care treatment. Echocardiography revealed a hypertrophic cardiomyopathy with low cardiac output. Laboratory investigations of blood carnitine, acyl-carnitines, amino acids, and organic acids in urine revealed no specific abnormalities. Lactate was constantly elevated in plasma, at 4–13 mmol/liter. With anticongestive therapy, the patient stabilized clinically, but cardiac hypertrophy was progressive. Severe muscular hypotonia and failure to thrive persisted. She had a poor weight gain despite high caloric intake. At age 4 mo, she died from heart failure. Muscle biopsy had been performed at age 2 wk. Histological examination showed lipid myopathy with lipid accumulation in both fiber types, prominent in type I fibers. There were no ragged red fibers on Gomori trichrome stain, but the oxidative enzyme stains nicotinamide adenine dinucleotide dehydrogenase, succinate dehydrogenase, and cytochrome c oxidase showed an abnormal mitochondrial network without relevant mitochondrial proliferation. Both lipid accumulation and the mitochondrial changes were confirmed by electron microscopy.
Patient 2, the elder sister of patient 1, was born at term, with a birth weight of 2,870 g (10th percentile), length of 53 cm (50th–90th percentile), head circumference of 33 cm (10th percentile), and Apgar scores of 8, 10, and 10 (at ages 1, 5, and 10 min, respectively). At age 10 h, she presented with muscular hypotonia, respiratory distress, metabolic acidosis (pH 6.9), and lactic acidosis (17 mmol/liter) that necessitated intensive-care treatment. Artificial ventilation was necessary for a period of 3 mo. The girl developed increasing hypertrophic cardiomyopathy. Metabolic workup revealed constantly elevated plasma lactate (5–14 mmol/liter) and an increased lactate:pyruvate ratio of 62. Blood carnitine, acyl-carnitines, amino acids, and organic acids in urine were normal. During her life, the child presented with severe muscular hypotonia and recurrent episodes of respiratory insufficiency that necessitated artificial ventilation. At age 9 mo, the child died from intractable low-output hypertrophic heart failure. Muscle biopsy had been performed at age 2 wk. No abnormalities were found via light microscopy. Electron microscopy revealed atypical and enlarged mitochondria, as well as an increased amount of lipid droplets indicative of a defect in fatty-acid oxidation or mitochondrial-energy metabolism. However, investigation of respiratory-chain enzymes showed no significant abnormalities. Activities in U/g protein (normal range) were as follows: for complex I, 20 (12–40); for complex II+III, 11 (6–25); for cytochrome c oxidase, 81 (90–281); and for citrate synthase, 79 (45–105).
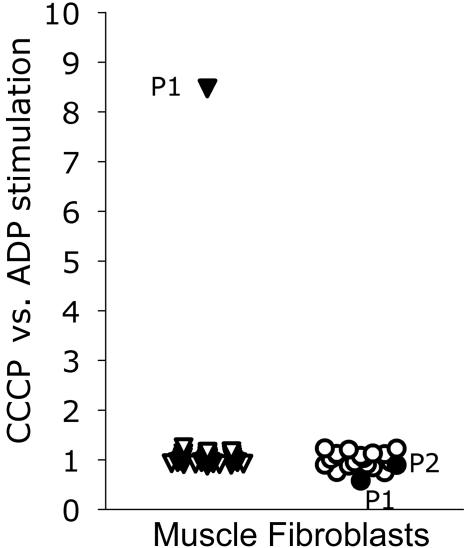
Investigation of intact mitochondria-enriched postnuclear supernatant from a fresh muscle biopsy specimen of patient 1 showed a severely reduced state 3 respiration of pyruvate, malate, and succinate (table 1).6 In the presence of the uncoupler CCCP (carbonyl cyanide m-chlorophenylhydrazone), a normal respiration rate was achieved (table 1 and fig. 2). This result was clear evidence of a defective synthesis of ATP in muscle. However, normal function of the respiratory chain (uncoupled oxidation) remained. As for patient 2, for patient 1, activity of respiratory-chain enzymes7 and pyruvate dehydrogenase8 was also normal, as was the oligomycin-sensitive ATPase of complex V7 (table 1). Analysis of pyruvate oxidation in digitonin-permeabilized fibroblasts showed normal ratios of CCCP versus ADP stimulated in both patients, conclusive for a normal ATP synthesis in fibroblasts in contrast to muscle (fig. 2). This was in accordance with the clinical presentation of cardiomyopathy and muscular hypotonia that was indicative of a tissue specificity of the defect.
Table 1. .
Functional and Enzymatic Investigations of Muscle Tissue[Note]
| Biochemical Measurements | Patient 1 | Controls |
| Substrate oxidation rate (nmol/h/mg protein): | ||
| [1–14C]pyruvate+malate+ADP | 45 | 263–900 |
| [1–14C]pyruvate+carnitine+ADP | 98 | 302–856 |
| [1–14C]pyruvate+malate (−ADP) | 35 | 32–102 |
| [U-14C]malate+pyruvate+malonate+ADP | 37 | 282–874 |
| [U-14C]malate+acetylcarnitine+malonate+ADP | 73 | 273–678 |
| [U-14C]malate+acetylcarnitine+arsenite+ADP | 52 | 156–378 |
| [1,4–14C]succinate+acetylcarnitine+ADP | 24 | 167–488 |
| [1–14C]pyruvate+malate+CCCP | 383 | 304–889 |
| [1–14C]pyruvate+malate+atractylate+ADP | 41 | 115–273 |
| Enzyme activity (unit/g protein): | ||
| Citrate synthase | 196 | 150–325 |
| Complex I | 63 | 28–76 |
| Complex I+III | 136 | 64–218 |
| Complex II | 80 | 39–102 |
| Complex II+III | 108 | 93–180 |
| Complex III | 622 | 426–762 |
| Cytochrome c oxidase | 539 | 452–889 |
| Oligomycin-sensitive ATPase (complex V) | 137 | 70–397 |
| Pyruvate dehydrogenase | 7.2 | 6.1–19.8 |
Note.— Postnuclear supernatant prepared from native, unfrozen muscle showed reduced activities in all ADP-stimulated oxidation reactions but was normal in the presence of the uncoupler CCCP. Analysis of respiratory-chain enzymes, ATPase, and pyruvate dehydrogenase revealed normal activities.
Figure 2. .
Respiratory-chain activity stimulated by CCCP, excluding ATP synthesis, in relation to oxidative phosphorylation stimulated by ADP, including the synthesis of ATP. This ratio is shown in muscle of patient 1 (P1) (blackened triangle), in 31 control individuals (unblackened triangles) (mean 1.01, SD 0.09), in fibroblasts of patient 1 and patient 2 (P2) (blackened circles), and in 18 controls (unblackened circles) (mean 1.01, SD 0.15).
Defective mitochondrial ATP synthesis (fig. 1) can be attributed to deficiencies of the F1F0-ATP synthase, the adenine nucleotide translocator (ANT), or the mitochondrial phosphate carrier. A possible defect of the F1F0-ATP synthase was ruled out by (1) normal oligomycin-sensitive ATPase activity, (2) normal levels of the enzyme on Blue Native polyarcylamide gels, and (3) sequence analysis of the ATP6 and ATP8 genes of ATP synthase subunits encoded by the mtDNA, which showed no pathogenic mutations. These investigations excluded all known presentations of ATP-synthase disorders. The search for mutations was next focused on the ANT whose isoforms are known to be expressed in a tissue-specific manner. The isoform encoded by the SLC25A4 (formerly known as “ANT1”) gene that is expressed in heart and muscle was sequenced,9 but no mutation could be detected.
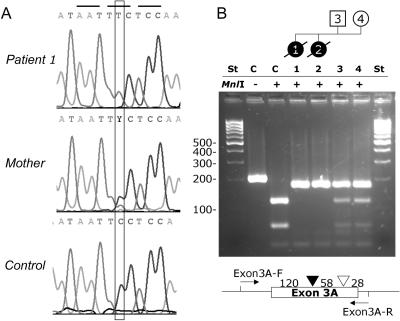
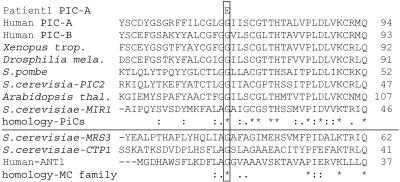
Finally, we investigated the mitochondrial phosphate carrier gene SLC25A3, located on chromosome 12q23.1. PCR amplification of the eight exons was performed with the following set of primers that started from the flanking intron or the noncoding parts of exon 1 or 8, respectively: for exons 1+2, 5′-ACACATCTTAGGACCCGGAG-3′ (forward) and 5′-ACTCGTCCTTCCCTGTCCT-3′ (reverse), with a product of 578 bp; for exon 3, 5′-TTCTTTCATTCCAGTGGCCT-3′ (forward) and 5′-CTAGCAGTTGTGATGTCTTCAAA-3′ (reverse), with a product of 583 bp; for exon 4, 5′-TATGTTAGCTGTTTGGTGCA-3′ (forward) and 5′-GGAACTACGCAAAGGAGTCC-3′ (reverse), with a product of 526 bp; for exon 5, 5′-GGACTCCTTTGCGTAGTTCC-3′ (forward) and 5′-GTGACAGAGCGAGACTTTGT-3′ (reverse), with a product of 506 bp; for exon 6, 5′-GTGTGCGGACATTTCTGTTA-3′ (forward) and 5′-AAAAAACGCCAATAATGGAA-3′ (reverse), with a product of 327 bp; and, for exons 7+8, 5′-TGGCTCTGTGAAGTTTTGTT-3′ (forward) and 5′-TCCTTTTGCACTTTCTTCAA-3′ (reverse), with a product of 481 bp. An annealing temperature of 54°C was used for all reactions. PCRs generating products <2,000 bp were performed with RedHot DNA polymerase (ABgene [HVD Life Sciences]). PCRs generating larger products and the cloning reactions were performed with AccuTaq DNA polymerase (Sigma-Aldrich), according to the manufacturer’s protocol. Sequencing (CEQ-8000 [Beckman Coulter]) revealed a homozygous missense mutation, c.215G→A (fig. 3), resulting in the substitution p.Gly72Glu in the two patients (GenBank accession number NM_005888). The parents are heterozygous carriers. The mutation was verified by restriction analysis (fig. 3). This mutation was not detectable in 376 chromosomes of Turkish control individuals. Remarkably, this mutation is localized in exon 3A, which is the alternatively spliced variant that produces the heart/muscle–specific isoform of PiC. The affected amino acid is located within the first transmembrane helix, and the substitution replaces a highly polar glutamic acid in lieu of the small neutral amino acid glycine. Alignment of PiC proteins from other eukaryotic organisms (fig. 4), performed with the ClustalW program,10 shows an absolute interspecies conservation of glycine at this position. Furthermore, this glycine of the first transmembrane helix is conserved in most other members of the mitochondrial carrier family11 (fig. 4), which consists of at least 46 members in humans,12 responsible for various carrier functions.
Figure 3. .
Sequence analysis (A) identified mutation c.215G→A resulting in the exchange p.Gly72Glu in exon 3A of the human mitochondrial PiC gene SLC25A3. The mutation was also shown by RFLP analysis (B) with the PCR primers Exon3A, 5′-TTCTTTCATTCCAGTGGCCT-3′ (forward) and 5′-AAAACAAACCTGCATTCTGC-3′ (reverse), with a PCR product of 206 bp. Digestion with MnlI (Fermentas) resulted in three fragments (119.5 bp, 58 bp, and 28.5 bp) for the normal allele and in two fragments (177.5 bp and 28.5 bp) in the mutated allele. St = DNA ladder, low range (Fermentas).
Figure 4. .
Sequence alignment of the mitochondrial PiC from different organisms (upper) and other members of the mitochondrial carrier (MC) family shows high conservation of the glycine 72 of human PiC isoform A. MRS3 = mitochondrial iron transporter; CTP1 = citrate transporter.
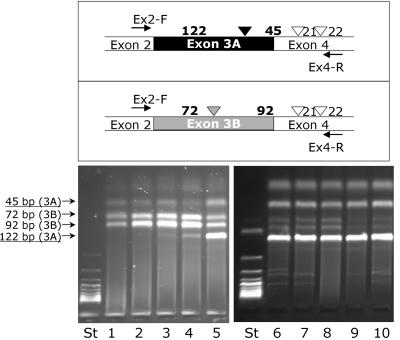
The presence of this mutation in exon 3A prompted us to investigate the tissue-specific expression of the two different isoforms of PiC in fibroblasts, myoblasts, and muscle (fig. 5). Isoform B was predominant in fibroblasts and also in undifferentiated myoblasts. After changing the culture medium to a differentiation medium (Promocell) for 10 d, a clear shift from isoform B to isoform A could be observed in differentiating myoblasts. Isoform A was present predominantly in muscle tissue, both in healthy control tissue and in tissue of patient 1 of our study. In all investigated muscle tissues, a small proportion of isoform B was detectable that may originate from either myoblasts or contaminating connective tissue or blood.
Figure 5. .
Investigation of mutually exclusive splicing of exons 3A and 3B of the human PiC in different tissues. DNA was amplified by PCR from cDNA with the primers Ex2, 5′-CGCCGCCGTGGAAG-3′ (forward) and 5′-ACCACGAACACCATCCTCTT-3′ (reverse), starting from the flanking exons 2 and 4, respectively. The resulting products were digested with Tru9I (Fermentas), which resulted in fragments of 122 bp, 45 bp, 21 bp, and 22 bp for isoform A and in 92 bp, 72 bp, 21 bp, and 22 bp for isoform B, respectively. Lanes 1 and 2 = control fibroblasts; lane 3 = fibroblasts from patient 1; lane 4 = myoblasts, undifferentiated from patient 1; lane 5 = myoblasts, differentiated from patient 1; lane 6 = muscle from patient 1; lanes 7–10 = muscle from controls.
To test the functional relevance of the identified mutation, we generated a strain of the yeast Saccharomyces cerevisiae that is devoid of mitochondrial PiC activity. As shown by Hamel and colleagues,3 S. cerevisiae has two distinct PiCs encoded by the MIR1 and PIC2 genes. Double mutants were created by crossing the respective deletion strains of the Euroscarf strain collection (Institute of Microbiology, Johann Wolfgang Goethe-University). The heterozygous diploids were sporulated, and the resulting colonies were tested by PCR analysis, to identify the double mutants. Double mutants grew normally on glucose-containing media but were unable to grow on 1% yeast extract, 2% peptone, and the nonfermentable carbon source glycerol (3%). Growth of the mir1-pic2 double mutant was restored by transformation with the MIR1 wild-type gene (plasmid YEp352-MIR1). The MIR1 sequence that included 1,000 bp upstream and 500 bp downstream cloned into the BamHI site of the episomal yeast plasmid after PCR amplification with BamHI site–tagged primers MIR1BamHI, 5′-CGGGATCCAAATATAAGCGTGCGCC-3′ (forward) and 5′-CGGGATCCAATGCAGAAGATGCGTCAAT-3′ (reverse), with a 2,628-bp product. To clone the human PiC isoforms in a yeast-compatible vector system, the MIR1 ORF in YEp352-MIR1 was replaced by an MluI site by PCR amplification with primers tagged with Mlu I sites (underlined) mir1MluI, 5′-GGCACGCGTGGTCTTGATTGATACATGC-3′ (forward) and 5′-GGCACGCGTATGAGACTTTTTGATCTTTGTAGT-3′ (reverse), with a 6,871-bp product. The PCR product was digested with MluI (Promega) and was ligated (T4 Ligase [Promega]), and the resulting plasmid YEp352-Δmir1-MluI was used for cloning and subsequent expression of the human PiC isoform A, as mentioned below. The human PiC gene was cloned from cDNA of muscle from either patient 1 or a control individual, after PCR amplification of the indicated coding regions flanked by MluI sites. The resulting clones were verified by sequencing, to exclude any mutations that may have been introduced by the cloning procedure.
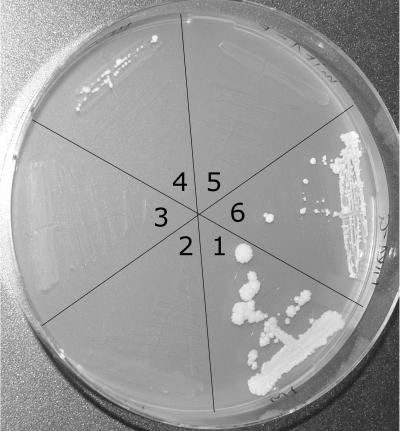
The human PiC isoform A was cloned without the first 49-aa targeting sequence that was shown to be not processed by yeast13; this sequence was replaced by a methionine codon. The cDNA was amplified with the following set of primers tagged with MluI sites: hPIC+49-MluI-F (5′-GATACGCGTATGTTCTCGTCCGTGGCG-3′) and hPIC-Mlu-R (5′-GGCACGCGTCTACTGAGTTAACCCAAGCTTTTTC-3′). The 959-bp product was cloned into the MluI site of the YEp352-Δmir1-MluI plasmid, was transformed14 into the yeast mir1-pic2 double mutant, and was selected for growth on uracil-free medium containing glucose (BIO101 [Eubio]). The transformed yeast mir1-pic2 double mutants expressing the human wild-type PiC gene were able to grow on glycerol medium, demonstrating functional complementation by the heterologous PiC. However, the efficiency was rather low, resulting in an ∼10 times slower growth rate compared with that of the YEp352-MIR1 plasmid harboring the authentic yeast carrier. No growth on nonfermentable glycerol medium was found when mir1-pic2 mutants were transformed with the mutant clone of the human PiC prepared from patient 1 (fig. 6), confirming that the mutated PiC is nonfunctional.
Figure 6. .
Expression of different PiC clones in the mutants of S. cerevisiae. Cells were grown for 3 wk on agarose plates containing 3% glycerol, 2% peptone, and 1% yeast extract. Segment 1 = wild-type strain BY4741; segment 2 = mir1-pic2 (mp) double mutant; segment 3 = mp hPICA-E (PiC-A from patient 1); segment 4 = mp hPICA-G (PiC-A from a control individual); segment 5 = mp yMIR1-E (site-specific mutation of the S. cerevisiae gene); segment 6 = mp yMIR1-G (wild-type form of S. cerevisiae).
Since the glycine residue that is mutated in the PiC of our patients is conserved in yeast PiCs (fig. 3), the corresponding glycine at position 24 in the yeast Mir1 protein was exchanged for a glutamic acid by site-specific mutagenesis. The plasmid YEp352-MIR1 was PCR amplified with mir-Nhe primers, 5′-TCGCgCTAGCCGaaGCCATAG-3′ (forward) and 5′-TCGGCTAGcGCGAATTTCATGTA-3′ (reverse) (changes from wild-type sequence in lowercase letters), which introduced an NheI site (underlined) without changing the ORF in addition to the desired Gly24Glu mutation (GGC→GAA, in bold). The resulting PCR product was digested with NheI (New England Biolabs), was ligated, was amplified, and was transformed into the yeast mir1-pic2 double mutant. The incorporated plasmid was analyzed by sequencing and showed the introduced changes but no other mutations. As shown in figure 6, the resulting transformed yeast could not restore growth on glycerol, demonstrating the functional relevance of the mutation.
Taken together, the missense mutation c.215G→A (p.Gly72Glu) found in our two patients affects a highly conserved amino acid in the mitochondrial phosphate carrier. The position is conserved in all known PiC sequences and, furthermore, in most other mitochondrial carrier proteins. The mutation introduces a large negatively charged amino acid instead of a small glycine. Expression of the wild-type protein of human PiC isoform A complemented the growth defect on glycerol of the yeast mir1-pic2 strain, which was not possible within the mutant form. Similarly, an exchange of the homologous glycine for a glutamic acid in the yeast Mir1 protein disabled growth complementation on the nonfermentable glycerol medium.
This is, to our knowledge, the first report of a disorder in the synthesis of ATP due to a mitochondrial phosphate–carrier deficiency. So far, mutations in the other mitochondrial carrier involved in the ATP synthesis, the ANT, have been reported. Most of the genetic defects of SLC25A4 (formerly ANT1) are heterozygous,15 but, recently, a homozygous mutation16 was identified. However, the pathomechanism of the ANT defects identified so far seems to be different from the phosphate–carrier deficiency described here and to be due mainly to an interference with the replication of the mtDNA. These patients are usually healthy early in life and acquire more and more deleted mtDNA. A high load of deletions of the mtDNA eventually results in a combined defect of the mitochondrially encoded enzymes of the oxidative phosphorylation. To prove whether the mtDNA is deleted in our patients, the whole mtDNA was amplified, in two distinct reactions, with the following set of primers (at an annealing temperature of 58°C): DLoop, 5′-CACCAGCCTAACCAGATTTCA-3′ (forward) and 5′-TGGTMCCCAAATCTGCTTCC-3′ (reverse), with a 15,679-bp product, as well as DLoop, 5′-CCAACCAAACCCCAAAGAC-3′ (forward) and 5′-TACTGCGACATAGGGTGCTC-3′ (reverse), with a 16,147-bp product. The resulting product showed the normal full length but no deleted mtDNA. Since mitochondrial PiC could influence the nucleotide pool in mitochondria, we analyzed mtDNA content in muscle of patient 1 but found no depletion.17 Therefore, no evidence of mtDNA changes due to mitochondrial PiC deficiency was detectable in our patients.
The main symptoms in our patients were cardiomyopathy, muscular hypotonia, and severe growth retardation combined with lactic acidosis. Neurologic involvement was not evident; however, brain magnetic resonance imaging was not performed for either of our patients. The clinical presentation is in accordance with the genetic defect that affects only the muscular isoform of the PiC created by alternative tissue-specific splicing. It can be assumed that mutations of the PiC outside the regions affected by alternative splicing would lead to a generalized disorder in mitochondrial-energy metabolism and a multisystemic clinical presentation.
Deficiency of the mitochondrial PiC cannot be identified by the analysis of the mitochondrial-energy metabolism in frozen tissue. Since the diagnosis of mitochondrial disorders is based on measurements of frozen tissue in many laboratories, this kind of defect can easily be missed. Therefore, at the moment, it is difficult to estimate the incidence of this novel disorder. We suggest that patients with clear clinical signs of a mitochondrial disorder but normal activities of respiratory-chain enzymes be re-evaluated. Functional investigation of intact mitochondria should be performed to detect disorders of ATP synthesis, including mitochondrial-PiC deficiency, and other mitochondrial transport defects.
Acknowledgments
We thank Anders Oldfors and Ali-Reza Moslemi for critical reading of the manuscript and helpful discussion. We thank Ingrid Vlasak, Gabriela Kronberger, and Alfred Klausegger for providing control samples. This work was supported by the Vereinigung zur Förderung der pädiatrischen Forschung und Fortbildung Salzburg; Jubiläumsfonds of the Oesterreichische Nationalbank project 10131 (to J.A.M.); the Austrian Federal Ministry for Education, Science and Culture (project GOLD–Genomics of Lipid-Associated Disorders in the framework of the GEN-AU program) (to S.D.K.); and Deutsche Forschungsgemeinschaft grant HO2505/2-1 (to R.H.). Human myoblast cultures were obtained from the Muscle Tissue Culture Collection (Friedrich-Baur-Institute), part of the German network on muscular dystrophies (MD-NET, service structure S1, 01GM0302) funded by the Bundesministerium für Bildung und Forschung, partner of EuroBioBank, funded by the European Union within the 5th framework (QLRT-2001-02769).
Web Resources
The accession number and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for SLC25A3 [accession number NM_005888])
- EuroBioBank, http://www.eurobiobank.org/index.htm
References
- 1.Skladal D, Halliday J, Thorburn DR (2003) Minimum birth prevalence of mitochondrial respiratory chain disorders in children. Brain 126:1905–1912 10.1093/brain/awg170 [DOI] [PubMed] [Google Scholar]
- 2.Palmieri F (2004) The mitochondrial transporter family (SLC25): physiological and pathological implications. Pflugers Arch 447:689–709 10.1007/s00424-003-1099-7 [DOI] [PubMed] [Google Scholar]
- 3.Hamel P, Saint-Georges Y, de Pinto B, Lachacinski N, Altamura N, Dujardin G (2004) Redundancy in the function of mitochondrial phosphate transport in Saccharomyces cerevisiae and Arabidopsis thaliana. Mol Microbiol 51:307–317 10.1046/j.1365-2958.2003.03810.x [DOI] [PubMed] [Google Scholar]
- 4.Huizing M, Ruitenbeek W, van den Heuvel LP, Dolce V, Iacobazzi V, Smeitink JA, Palmieri F, Trijbels JM (1998) Human mitochondrial transmembrane metabolite carriers: tissue distribution and its implication for mitochondrial disorders. J Bioenerg Biomembr 30:277–284 10.1023/A:1020501021222 [DOI] [PubMed] [Google Scholar]
- 5.Fiermonte G, Dolce V, Palmieri F (1998) Expression in Escherichia coli, functional characterization, and tissue distribution of isoforms A and B of the phosphate carrier from bovine mitochondria. J Biol Chem 273:22782–22787 10.1074/jbc.273.35.22782 [DOI] [PubMed] [Google Scholar]
- 6.Bookelman H, Trijbels JM, Sengers RC, Janssen AJ, Veerkamp JH, Stadhouders AM (1978) Pyruvate oxidation in rat and human skeletal muscle mitochondria. Biochem Med 20:395–403 10.1016/0006-2944(78)90089-3 [DOI] [PubMed] [Google Scholar]
- 7.Mayr JA, Paul J, Pecina P, Kurnik P, Forster H, Fotschl U, Sperl W, Houstek J (2004) Reduced respiratory control with ADP and changed pattern of respiratory chain enzymes as a result of selective deficiency of the mitochondrial ATP synthase. Pediatr Res 55:988–994 10.1203/01.pdr.0000127016.67809.6b [DOI] [PubMed] [Google Scholar]
- 8.Sperl W, Trijbels JM, Ruitenbeek W, van Laack HL, Janssen AJ, Kerkhof CM, Sengers RC (1993) Measurement of totally activated pyruvate dehydrogenase complex activity in human muscle: evaluation of a useful assay. Enzyme Protein 47:37–46 [DOI] [PubMed] [Google Scholar]
- 9.Kiechl S, Horvath R, Luoma P, Kiechl-Kohlendorfer U, Wallacher-Scholz B, Stucka R, Thaler C, Wanschitz J, Suomalainen A, Jaksch M, et al (2004) Two families with autosomal dominant progressive external ophthalmoplegia. J Neurol Neurosurg Psychiatry 75:1125–1128 10.1136/jnnp.2003.025890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31:3497–3500 10.1093/nar/gkg500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wohlrab H (2005) The human mitochondrial transport protein family: identification and protein regions significant for transport function and substrate specificity. Biochim Biophys Acta 1709:157–168 10.1016/j.bbabio.2005.07.003 [DOI] [PubMed] [Google Scholar]
- 12.Haitina T, Lindblom J, Renstrom T, Fredriksson R (2006) Fourteen novel human members of mitochondrial solute carrier family 25 (SLC25) widely expressed in the central nervous system. Genomics 88:779–790 10.1016/j.ygeno.2006.06.016 [DOI] [PubMed] [Google Scholar]
- 13.Zara V, Palmieri F, Mahlke K, Pfanner N (1992) The cleavable presequence is not essential for import and assembly of the phosphate carrier of mammalian mitochondria but enhances the specificity and efficiency of import. J Biol Chem 267:12077–12081 [PubMed] [Google Scholar]
- 14.Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaukonen J, Juselius JK, Tiranti V, Kyttala A, Zeviani M, Comi GP, Keranen S, Peltonen L, Suomalainen A (2000) Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science 289:782–785 10.1126/science.289.5480.782 [DOI] [PubMed] [Google Scholar]
- 16.Palmieri L, Alberio S, Pisano I, Lodi T, Meznaric-Petrusa M, Zidar J, Santoro A, Scarcia P, Fontanesi F, Lamantea E, et al (2005) Complete loss-of-function of the heart/muscle-specific adenine nucleotide translocator is associated with mitochondrial myopathy and cardiomyopathy. Hum Mol Genet 14:3079–3088 10.1093/hmg/ddi341 [DOI] [PubMed] [Google Scholar]
- 17.Reichenbach J, Schubert R, Horvath R, Petersen J, Futterer N, Malle E, Stumpf A, Gebhardt BR, Koehl U, Schraven B, et al (2006) Fatal neonatal-onset mitochondrial respiratory chain disease with T cell immunodeficiency. Pediatr Res 60:321–326 10.1203/01.pdr.0000233252.60457.cf [DOI] [PubMed] [Google Scholar]