Abstract
Broad aspects of Caenorhabditis elegans life history, including larval developmental timing, arrest at the dauer diapause, and longevity, are regulated by the nuclear receptor DAF-12. Endogenous DAF-12 ligands are 3-keto bile acid-like steroids, called dafachronic acids, which rescue larval defects of hormone-deficient mutants, such as daf-9/cytochrome P450 and daf-36/Rieske oxygenase, and activate DAF-12. Here we examined the effect of dafachronic acid on pathways controlling lifespan. Dafachronic acid supplementation shortened the lifespan of long-lived daf-9 mutants and abolished their stress resistance, indicating that the ligand is “proaging” in response to signals from the dauer pathways. However, the ligand extended the lifespan of germ-line ablated daf-9 and daf-36 mutants, showing that it is “antiaging” in the germ-line longevity pathway. Thus, dafachronic acid regulates C. elegans lifespan according to signaling state. These studies provide key evidence that bile acid-like steroids modulate aging in animals.
Keywords: aging, DAF-12, hormone, dafachronic acid, germ-line longevity
Endocrine systems coordinate many facets of animal metabolism, reproduction, and homeostasis, ensuring the proper response to changes in physiology or environment. More recently, hormones have also been shown to govern metazoan aging. Notably, a mild reduction of insulin/IGF1 signaling increases stress resistance and longevity in diverse species across taxa (1). These studies also imply that other hormones impact lifespan. Nuclear hormone receptors are transcription factors that respond to lipophilic hormones such as steroids, fatty acids, and retinoids to regulate gene expression, and are well poised to coordinate global life history traits. Indeed, genetic studies in worms and flies have implicated such molecules in lifespan regulation, but how they do so is not well understood (2–7).
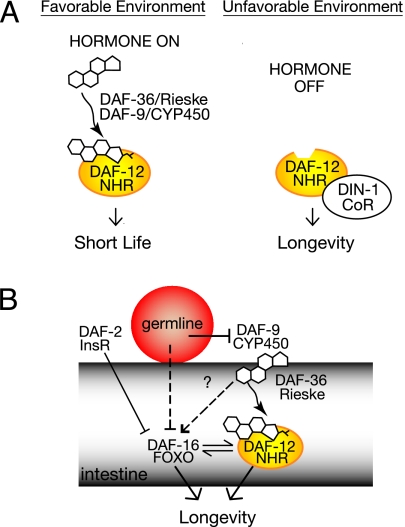
DAF-12 is one such nuclear receptor in Caenorhabditis elegans. Homologous to vertebrate vitamin D and LXR receptors (8), DAF-12 controls the choice between reproductive growth and arrest at a long-lived, alternate third larval stage, the dauer diapause, formed under harsh environmental conditions (9). It also specifies third larval temporal fates in the heterochronic pathway (10), a circuit that regulates developmental timing. Finally, DAF-12 and related components modulate organismal lifespan in at least two contexts (2–6): (i) in response to inputs for dauer diapause and (ii) in response to signals from the germ line. Recently, the endogenous ligands of DAF-12 have been discovered to be 3-keto bile acid-like steroids, called Δ4- and Δ7-dafachronic acids (11) and related metabolites (12), enabling a direct test of whether these ligands modulate nematode longevity and stress resistance. Here, we specifically examined Δ4-dafachronic acid.
Results
In the dauer pathways, environmental cues (temperature, dauer pheromone, food, and cholesterol) are integrated by insulin/IGF1 and TGF-β signaling, which then converge on daf-9/cytochrome P450 (CYP450) to regulate hormone production. daf-9/CYP450 is the functional ortholog of the bile acid-producing cytochrome P450 CYP27 of mammals, catalyzing oxidation of the sterol side chain to the 26 acid (11). daf-9-null mutants uncouple upstream inputs from hormone production, resulting in the absence of DAF-12 ligands under all circumstances. Such mutants constitutively arrest as partially remo deled dauer-like larvae, a proportion of which recover to adults that are sterile and modestly long-lived, with mean lifespan extension ranging from 19% to 41% at 15°C (Table 1) (5, 6). By contrast, daf-9 hypomorphs, in which daf-12 is presumably partially liganded, have normal or even shortened lifespans. Because daf-12-null mutants suppress daf-9 longevity, it is hypothesized that a hormone-regulated switch governs C. elegans lifespan: Long life stems from the activity of the unliganded receptor, whereas normal lifespan arises from the liganded receptor (5, 6) (Fig. 1A). To test this hypothesis, we fed daf-9 mutants the DAF-12 ligand, Δ4-dafachronic acid, and measured the resultant lifespans of hormone-supplemented animals. A concentration of 250 nM was chosen because this level is sufficient to give robust rescue of larval phenotypes, and also approximates the endogenous concentration of 200 nM estimated in ref. 11.
Table 1.
daf-9 longevity is modulated by ligand- and tissue-specific daf-9(+) expression
| Genotype | Temperature, °C | Ligand or daf-9(+) expression | Lifespan, days |
No. of animals (no. of exps) | P value | |
|---|---|---|---|---|---|---|
| Mean ± SD | Maximum ± SD | |||||
| N2 (wild type) | 15 | — | 27 ± 1 | 41 ± 2 | 102 (2) | |
| Δ4 | 27 ± 2 | 41 ± 2 | 131 (2) | 0.38*, 0.88† | ||
| Δ4(d) | 26 | 36 | 65 (1) | 0.37* | ||
| daf-9(dh6) | 15 | — | 33 ± 3 | 57 ± 4 | 148 (2) | <0.00001* |
| Δ4 | 27 ± 1 | 41 ± 2 | 182 (2) | 0.083‡, <0.00001§ | ||
| Δ4(d) | 34 | 42 | 166 (1) | <0.00001†, 0.30§ | ||
| daf-9(e1406)§§ | 15 | — | 34 ± 3 | 53 ± 1 | 97 (2) | <0.00001* |
| Δ4 | 26 ± 1 | 38 ± 2 | 225 (2) | 0.14‡, <0.00001§ | ||
| Δ4(d) | 31 | 42 | 174 (1) | <0.00001†, 0.00013§ | ||
| e1406¶¶ | 15 | — | 38 | 54 | 55 (1) | <0.00001* |
| Δ4 | 28 | 39 | 115 (1) | 0.11‡, <0.00001§ | ||
| Δ4(d) | 28 | 39 | 160 (1) | 0.027†, <0.00001§ | ||
| N2 | 15 | 31 ± 0 | 40 ± 2 | 208 (3) | ||
| N2 dhEx203 | XXX | 30 | 40 | 38 (1) | 0.62¶ | |
| N2 dhEx294 | hyp | 31 | 42 | 106 (1) | 0.54¶ | |
| e1406§§ | 15 | 35 ± 2 | 61 ± 1 | 198 (3) | <0.00001¶ | |
| e1406 dhEx203 | XXX | 31 ± 1 | 44 ± 1 | 174 (2) | 0.13‖, <0.00001** | |
| e1406 dhEx294 | hyp | 31 ± 1 | 43 ± 2 | 212 (2) | 0.54‖, <0.00001** | |
| N2 | 22.5 | — | 18 ± 2 | 25 ± 2 | 201 (3) | |
| Δ4 | 21 ± 2 | 32 ± 2 | 236 (3) | <0.00001* | ||
| daf-9(rh50) | 22.5 | — | 12 ± 0 | 20 ± 2 | 129 (2) | <0.00001* |
| Δ4 | 18 ± 0 | 30 ± 0 | 140 (2) | <0.00001‡, <0.00001§ | ||
| daf-2(e1368) | 22.5 | — | 32 ± 4 | 42 ± 3 | 206 (3) | <0.00001* |
| Δ4 | 35 ± 2 | 47 ± 2 | 144 (3) | <0.00001‡, 0.000055§ | ||
| daf-2(e1370) | 22.5 | — | 33 ± 1 | 63 ± 10 | 77 (2) | <0.00001* |
| Δ4 | 35 ± 6 | 68 ± 2 | 59 (2) | <0.00001‡, 0.17§ | ||
| N2 | 20 | — | 20 ± 3 | 39 ± 2 | 524 (3) | |
| Δ4 | 20 ± 2 | 40 ± 3 | 534 (3) | 0.029* | ||
| daf-36(k114) | 20 | — | 13 ± 1 | 29 ± 1 | 289 (2) | <0.00001* |
| Δ4 | 16 ± 3 | 53 ± 14 | 401 (2) | <0.00001‡, <0.00001§ | ||
| daf-9(rh50) | 20 | — | 13 ± 1 | 27 ± 6 | 291 (2) | <0.00001* |
| Δ4 | 15 ± 0 | 35 ± 6 | 239 (2) | <0.00001‡, <0.00001§ | ||
| daf-12(rh61rh411) | 20 | — | 14 ± 1 | 31 ± 2 | 322 (2) | <0.00001* |
| Δ4 | 14 ± 1 | 39 ± 8 | 344 (2) | <0.00001‡, 0.44§ | ||
| glp-1(e2141) | 20 | — | 23 ± 2 | 43 ± 1 | 487 (2) | <0.00001* |
| Δ4 | 22 ± 4 | 43 ± 1 | 589 (2) | <0.00001‡, 0.52§ | ||
| e2141 k114 | 20 | — | 16 ± 2 | 35 ± 1 | 384 (2) | <0.00001*, <0.00001†† |
| Δ4 | 22 ± 4 | 50 ± 7 | 501 (2) | <0.00001‡, <0.00001§, 0.38‡‡ | ||
| e2141 rh50 | 20 | — | 18 ± 3 | 39 ± 1 | 241 (2) | 0.86*, <0.00001†† |
| Δ4 | 23 ± 1 | 64 ± 5 | 429 (2) | <0.00001‡, <0.00001§, 0.029‡‡ | ||
| e2141 rh61rh411 | 20 | — | 16 ± 1 | 41 ± 1 | 482 (2) | <0.00001*, <0.00001†† |
| Δ4 | 16 ± 2 | 73 ± 23 | 353 (2) | <0.00001‡, 0.39§, <0.00001‡‡ | ||
The ligand (Δ4) was added during larval development and adulthood. — indicates that only vehicle (100% EtOH) was added. Δ4(d) indicates that ligand was added during larval development only. daf-9 cDNA was tissue-specific expressed in XXX cells (XXX) or in the hypodermis (hyp). SDs were calculated from the average of the mean or maximum of individual experiments. P values were determined by using the log rank test (Mantel–Cox) on the pooled data of all experiments performed within a set. dh6 was derived from daf-9(dh6) dhEx24.
*Compared to N2 worms without ligand (—).
‡Compared to N2 worms exposed to the Δ4 ligand during larval development and adulthood (Δ4).
†Compared to N2 worms exposed to the Δ4 ligand during larval development only [Δ4(d)].
§Compared to the same strain without ligand (—).
¶Compared to N2 worms without daf-9(+) expression constructs.
‖Compared to N2 worms expressing daf-9(+) in the same tissue.
**Compared to the same strain without daf-9(+) expression constructs.
††Compared to glp-1 worms without ligand (—).
‡‡Compared to glp-1 worms exposed to the Δ4 ligand (Δ4).
§§e1406 was derived from daf-9(e1406) dhEx203.
¶¶e1406 was derived from daf-9(e1406)/lon-2(e678).
Fig. 1.
Dauer and germ-line longevity pathways. Δ4-dafachronic acid inhibits longevity in the dauer pathways but promotes it in the germ-line longevity pathway, showing a dependence on signaling context. Germ-line signaling is DAF-16/FOXO-dependent, whereas dauer signaling can be rendered DAF-16/FOXO-independent. Both contexts require DAF-12/nuclear hormone receptor. (A) Dauer longevity. Liganded DAF-12 promotes reproductive development and short life, whereas unliganded DAF-12 together with corepressor DIN-1 promote stress resistance and longevity. (B) Germ-line longevity. In the absence of signals from the germ line, liganded DAF-12, together with nuclear localized DAF-16/FOXO, promote longevity.
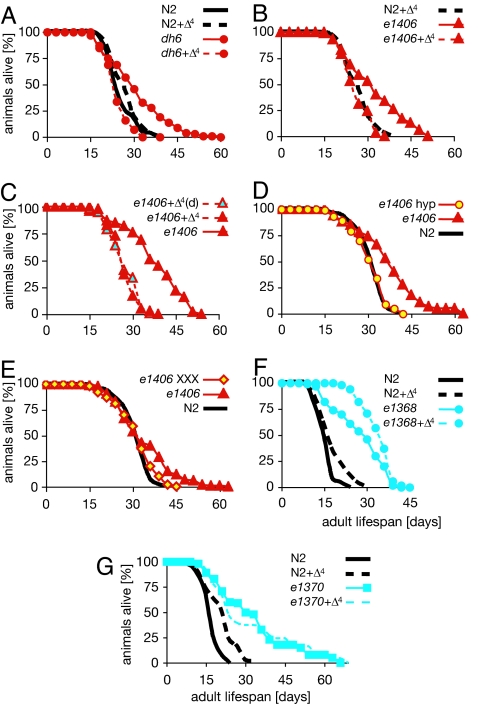
Whereas daf-9(dh6)-null mutants lived significantly longer than wild type (P < 0.00001) when exposed to the ethanol vehicle alone (mean = 33 ± 3, maximum = 57 ± 4), mutants supplemented with 250 nM Δ4-dafachronic acid during larval development and adulthood lived lifespans comparable to N2 wild type (P = 0.083) (mean = 27 ± 1, maximum = 41 ± 2) (Table 1 and Fig. 2A). Likewise, the strong loss-of-function daf-9 mutant, e1406, gave comparable results (Table 1 and Fig. 2 B and C) even when derived from a variety of genetic backgrounds (Table 1). This restoration of normal lifespan to daf-9 mutants was unlikely to be due to compound toxicity, because treated animals developed rapidly, readily bypassed dauer arrest and became normal-looking, gravid adults that were indistinguishable from wild type (11). Moreover, wild-type N2 animals and other genotypes treated with the DAF-12 ligand had lifespans with normal variability, on occasion somewhat shorter or longer, making life-shortening toxicity unlikely (Fig. 2, Table 1, and supporting information (SI) Table 2]. As expected, daf-12(rh61rh411)-null mutants had similar lifespans independent of the presence or absence of hormone (Fig. 3F, Table 1, and SI Table 2), suggesting that the hormone works through daf-12.
Fig. 2.
daf-9 longevity is hormone-dependent. The lifespan of adult animals was measured (in days) on OP50 bacterial lawns in the absence (solid line) or presence (dashed line) of added ligand (250 nM). Ligand was added during larval development and adulthood (Δ4) or during larval development only [Δ4(d)]. The experiments were performed at 15°C unless noted otherwise. Individual experiments are shown. Averages of mean and maximum lifespans are shown in Table 1. (A) Lifespan of wild-type N2 and daf-9(dh6). (B) Lifespan daf-9(e1406). (C) Lifespan of daf-9(e1406) when hormone was added during larval development only. (D) Lifespan of daf-9(e1406) in the presence of hypodermal-expressed wrt-1p::daf-9::gfp (hyp) during larval development. (E) Lifespan of daf-9(e1406) in the presence of daf-9::gfp expressed throughout life in the XXX neuroendocrine cells (XXX). (F) Lifespan of daf-2(e1368) at 22.5°C. (G) Lifespan of daf-2(e1370) at 22.5°C.
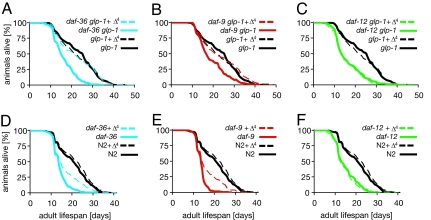
Fig. 3.
Lifespan in the germ-line longevity pathway is ligand-dependent. Strains were cultured at 25°C during larval development, and adult aging was carried out at 20°C in the absence (solid line) or presence (dashed line) of added ligand (250 nM). Pooled data of two experiments are shown. Averages of mean and maximum lifespans are shown in Table 1. (A) Lifespan of glp-1(e2141) and daf-36(k114) glp-1(e2141). (B) Lifespan of glp-1(e2141) and daf-9(rh50) glp-1(e2141). (C) Lifespan of glp-1(e2141) and daf-12(rh61rh411) glp-1(e2141). (D) Lifespan of wild-type N2 and daf-36(k114). (E) Lifespan of N2 and daf-9(rh50). (F) Lifespan of N2 and daf-12(rh61rh411).
We then asked, when is hormone required to regulate longevity? To do this, we fed hormone to daf-9 mutants during larval development and then shifted them to media lacking hormone during adulthood. Similar to wild type, daf-9 animals exposed to hormone during larval development bypassed the dauer diapause, and lived shorter than daf-9 without hormone (Fig. 2C). In some experiments, the mean lifespan was somewhat higher, but the maximum was usually the same as wild type (Table 1). Consistent with this result, we found that larval expression of daf-9(+) under control of the wrt-1 promoter in the hypodermis, an endogenous daf-9 endocrine tissue, restored a nearly normal lifespan to daf-9 mutants (Fig. 2D and Table 1). These animals resembled animals exposed their entire lives to daf-9(+) expressed in the XXX neuroendocrine cells (Fig. 2E and Table 1), another daf-9-expressing tissue (5, 6, 13). Taken together, these results suggest that larval exposure to hormone can impact adult longevity.
During larval development, daf-9 and daf-12 integrate signals from the insulin/IGF1 pathway to mediate dauer formation (5, 6, 14, 15). Consistent with a downstream role, daf-9 overexpression or hormone supplementation of daf-2/insulin/IGF1 receptor mutants readily prevents constitutive dauer formation of weak daf-2 alleles (e.g., e1368) (11, 14, 15). Such daf-2 alleles also induce longevity in adult animals, doubling the lifespan by activating daf-16/FOXO. Moreover, daf-2 and daf-16 regulate lifespan by downstream cell nonautonomous signals (16–18). Given the downstream role of DAF-12 ligands for dauer diapause, we asked whether similar relationships would hold for adult lifespan regulation. On the contrary, we found that hormone supplementation could not suppress daf-2 longevity (Fig. 2 F and G and Table 1). In fact, added ligand appeared to enhance daf-2(e1368) longevity, suggesting that hormone may maximize daf-16/FOXO-dependent life extension in some contexts. Thus, dafachronic acid can bypass daf-16/FOXO for dauer formation but not for longevity, and in this context, daf-2/daf-16 regulate lifespan somewhat autonomously from hormone signaling.
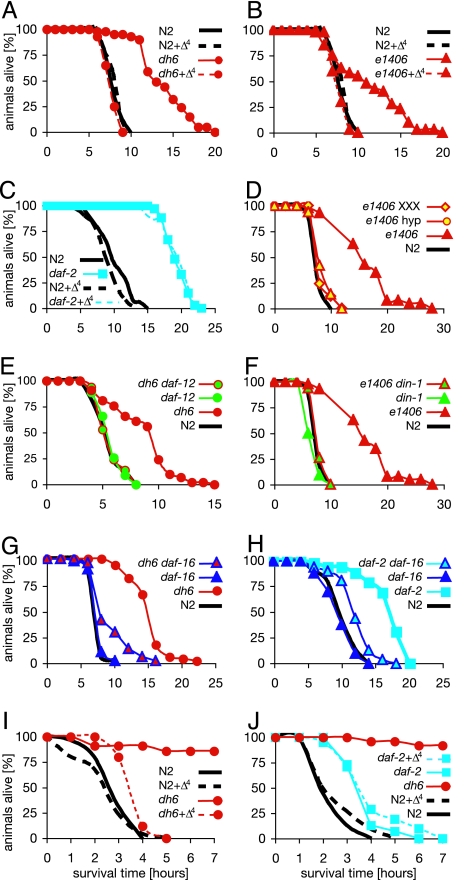
Increased longevity often correlates with resistance to heat and oxidative stress (19, 20). To test this correlation, we examined the survival of daf-9 mutants exposed to such stress in the presence or absence of hormone. Consistent with increased somatic endurance, we found that daf-9 adults exhibited significantly stronger resistance to heat stress at 35°C compared with wild type, living 38–125% longer (Fig. 4A, B, and D–G and SI Table 3). Such intrinsic thermotolerance was similar to that observed in mutants of the insulin/IGF1 receptor daf-2, which are thermotolerant by 58–100%. The resistance of daf-9 mutant worms was substantially hormone-dependent, whereas daf-2(e1370) resistance was not (Fig. 4 A–C). Similarly, daf-9(e1406) mutants rescued by a daf-9(+) transgene expressed in the hypodermis or XXX cells (Fig. 4D) were as susceptible to heat stress as wild type. Similar to dauer formation and lifespan, daf-9 thermotolerance was also dependent on daf-12 as well as din-1/SHARP corepressor (Fig. 4 E and F) (21), which forms a complex with daf-12 to repress transcription in the unliganded state (Fig. 1A) (11). In addition, we found that resistance was partly dependent on daf-16/FOXO, which mediates stress resistance and longevity of insulin/IGF1 signaling (Fig. 4 G and H).
Fig. 4.
daf-9 stress resistance depends on ligand, daf-12(+) and din-1(+). (A–H) Thermotolerance. Young adult animals were shifted to 35°C, and survival was measured over time in the absence (solid line) or presence (dashed line) of added ligand (250 nM). Individual experiments are shown. Mean and maximum survival parameters are shown in SI Table 3. (I and J) Oxidative stress resistance. Young adult animals were shifted to plates containing 4.4–8.4 mM H2O2, and survival was measured over time in the absence (solid line) or presence (dashed line) of added ligand (250 nM). Individual experiments are shown. Mean and maximum survival parameters of individual experiments are shown in SI Table 4. (A) Thermotolerance of wild-type N2 and daf-9(dh6). (B) Thermotolerance of N2 and daf-9(e1406). (C) Thermotolerance of N2 and daf-2(e1370). (D) Thermotolerance of daf-9(e1406) in the presence of hypodermal-expressed wrt-1p::daf-9::gfp (hyp) during larval development and daf-9::gfp expressed throughout life in the XXX neuroendocrine cells (XXX). (E) Thermotolerance of daf-9(dh6) is abolished by daf-12(rh61rh411). (F) Thermotolerance of daf-9(e1406) is abolished by din-1(dh127). (G) Thermotolerance of daf-9(dh6) is reduced by daf-16(mgDf50). (H) Thermotolerance of daf-2(e1370) is reduced by daf-16(mg47). (I) Oxidative stress resistance of N2 and daf-9(dh6). (J) Oxidative stress resistance of N2 and daf-2(e1370).
We then examined resistance to oxidative stress. We exposed animals to millimolar levels of hydrogen peroxide and again found that daf-9 mutants were substantially resistant compared with wild type (Fig. 4 I and J and SI Table 4). Moreover, hormone replacement restored normal resistance (Fig. 4I), as did transgene rescue (SI Fig. 6 A and B). The resistance of daf-9 mutants was also daf-12- and din-1-dependent (SI Fig. 6 C and D). These results reveal that hormone-deficient animals are more stress-resistant in a manner dependent on daf-12/din-1 corepressor complexes and that hormone-supplemented animals have normal or decreased resistance. Similar to thermotolerance resistance and lifespan, daf-2(e1370) mutants were not affected by ligand for their oxidative stress resistance (Fig. 4J).
daf-9(+) and daf-12(+) are also required for longevity in another context, that of the germ-line longevity pathway (4, 6). In this pathway, unknown signals originating from the gonad interact with specific genotypes to influence lifespan. In the absence of germ-line stem cells, animals live 50–60% longer than wild type (22), most likely due to a systemic signal. Longevity is not due to sterility, because animals lacking both somatic gonad and germ line have normal lifespans, suggesting that these tissues produce opposing signals that govern long life. The longevity of germ-lineless animals depends on the hormone biosynthetic genes daf-36/Rieske-like oxygenase and daf-9/CYP450, as well as the transcription factors daf-12/nuclear hormone receptor and daf-16/FOXO: in these mutant backgrounds, germ-line-ablated animals are no longer long-lived (4, 6, 23), implicating lipophilic hormones as part of a systemic signal. Germ-line longevity is additive with lifespan extension due to reduced daf-2/insulin/IGF1 receptor, revealing that it works independently of insulin/IGF1 signal transduction. Moreover, germ-line absence causes DAF-16 translocation to the intestinal nuclei in a manner that is daf-9(+)-dependent (24, 25). Hence, in contrast to dauer signaling, DAF-12 ligands are predicted to be required to attain maximal lifespan extension in the germ-line longevity pathway (Fig. 1B).
To test this hypothesis, we examined the influence of hormonal manipulation on germ-line longevity. To induce germ-line ablation, we used the glp-1 temperature-sensitive allele, e2141, which has normal germ cell development at 15°C but arrests it at 25°C and extends adult lifespan (22) (Table 1 and SI Table 2). To reduce hormonal signals, we used the daf-36/Rieske-like oxygenase-null mutant k114 or the weak daf-9 mutant rh50. Both mutants are somewhat shorter lived than wild type, presumably because DAF-12 is not fully activated, and both abolish the longevity of animals whose germ lines have been removed by laser microsurgery (6, 23). Consistent with previous results, daf-36 glp-1 (mean = 16 ± 2) and daf-9 glp-1 (mean = 18 ± 3) double mutants have significantly shortened lifespans (P < 0.00001) compared with glp-1 alone (mean = 23 ± 2) (Fig. 3 A and B and Table 1). Importantly, hormone supplementation of the double mutants restored longevity to that of similarly treated glp-1 mutants (P > 0.025), suggesting that Δ4-dafachronic acid is required to extend lifespan in the germ-line longevity pathway (Fig. 3 A and B). Similar trends were seen whether animals were cultured at 25°C for their whole lives or downshifted to 20°C during the adult phase, although the variability between experiments was larger at 25°C (SI Fig. 7 and Table 2). By contrast, glp-1 daf-12- and daf-12-null mutants were unaffected by the addition of hormone (Fig. 3 C and F, Table 1, and SI Table 2), revealing a strict dependence on the nuclear receptor. Hormone replacement of daf-36(k114) and daf-9(rh50) mutants alone partially or fully restored lifespan to that of wild type (Fig. 3 D and E, Table 1, and SI Table 2). Conceivably, partial rescue could result from the effect of accumulated biosynthetic intermediates in mutants blocked in hormone production or from imprecise levels of hormone.
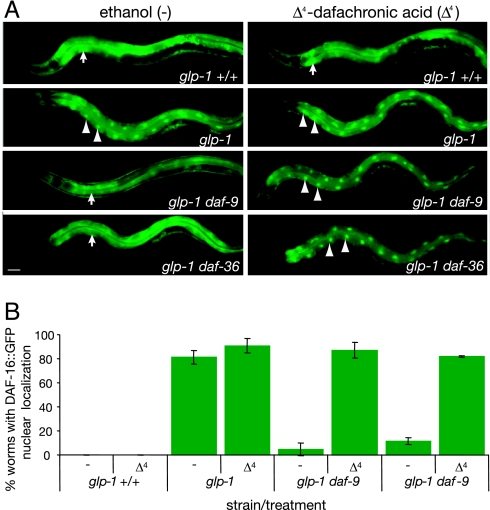
As another measure of activity within the germ-line longevity pathway, we examined the status of DAF-16/FOXO nuclear localization. In glp-1 animals, DAF-16::GFP localizes to intestinal nuclei and correlates with longevity. By contrast, in the glp-1daf-9 background, DAF-16 nuclear localization is reduced, suggesting that DAF-16 nuclear action depends on DAF-12 ligands (24, 25). We asked whether daf-36 mutants would behave as daf-9 and whether DAF-16 localization was susceptible to ligand. Indeed, we found that DAF-16 nuclear localization was reduced in daf-9 or daf-36 mutants (4.6, 11.6%), but fully restored (87.1, 82%) when animals were given ligand (Fig. 5). Thus, hormone replacement is required to produce the full extent of DAF-16/FOXO and DAF-12 activity induced by germ-line ablation.
Fig. 5.
DAF-16::GFP localization to the intestinal nuclei in the germ-line longevity pathway is ligand-dependent. Animals were grown in the presence or absence of ligand at 25°C. DAF-16::GFP nuclear localization was scored on day 1 of adulthood. (A) Photomicrographs of DAF-16::GFP localization in the indicated genotypes, on ethanol vehicle (Left) or ligand (Right). Arrowheads indicate intestinal nuclei, arrows indicate cytoplasmic localization. (Scale bar: 10 μm.) (B) Bar graphs quantifying percentage of worms with DAF-16::GFP nuclear localization. Error bars indicate standard deviations from at least two independent trials (n > 160 animals).
Discussion
The studies reported here provide crucial evidence that bile acid-like steroids can modulate metazoan stress resistance and lifespan. In worms, Δ4-dafachronic acid may be permissive rather than instructive, because its precise effect depends on signaling context (Fig. 1). Increased longevity and somatic endurance in the absence of ligand [i.e., daf-9(-) longevity] may reflect the response to environmental adversity, such as limited dietary sterols or food. Conversely, production of bile acid-like ligands may signal the fed state, promoting reproduction and altered metabolism. Longevity in the absence of the germ line may reveal how physiologic signals emanating from distinct tissues communicate to coordinate events throughout the body. Such coordination evidently relies on the production of dafachronic acid, which regulates DAF-16/FOXO translocation. Conceivably, other lipophilic hormones can also regulate animal longevity. Interestingly, in mammals, serum bile acids regulate triglyceride, cholesterol, energy, and glucose homeostasis, pointing toward important signaling roles (26). Moreover, in long-lived Ames dwarf mice, rate-limiting enzymes for bile acid synthesis are down-regulated (27). Conceivably, altered bile acid metabolism can impact longevity in this model. Notably, on the basis of known activities of bile acid metabolism (28), the dafachronic acids and related metabolites could be present in mammals. Indeed, the mammalian LXR, as well as its C. elegans homolog DAF-12, are reportedly activated by cholestenoic acid derivatives (12, 29). Because many of the components of these circuits are evolutionarily conserved, some of the findings here may ultimately yield insights into hormonal regulation of mammalian stress resistance and aging.
Methods
C. elegans Stock and Nematode Culture.
Nematodes were cultured at 20°C on NG agar plates with the E. coli strain OP50, unless indicated otherwise. The following strains were used: N2, daf-9(rh50), daf-9(dh6) dhEx24, daf-9(dh6) dhEx203, daf-9(e1406) dhEx203, daf-9(e1406)/lon-2(e678), daf-9(e1406) dhEx294, daf-9(rh50), daf-12(rh61rh411), daf-9(dh6) daf-12(rh61rh411), daf-9(e1406) daf-12(rh61rh411), daf-36(k114), din-1(dh127), daf-9(e1406) din-1(dh127), daf-2(e1370), daf-2(e1368), daf-16(mgDf50), daf-9(dh6) daf-16(mgDf50), daf-2(e1370) daf-16(mg47), glp-1(2141), daf-36(k114) glp-1(e2141), daf-9(rh50) glp-1(e2141), daf-12(rh61rh411) glp-1(e2141), daf-16(mu86) muEx248(Pdaf-16::gfp::daf-16), glp-1(2141) daf-16(mu86) muEx248, daf-36(k114) glp-1(e2141) daf-16(mu86) muEx248, daf-9(rh50) glp-1(e2141) daf-16(mu86) muEx248.
Array dhEx203 was made from dr434 (5), which consists of 3.03 kb of daf-9 promotor and isoform B daf-9 cDNA fused with gfp (14). For hypodermal-specific expression, 3.18 kb of the wrt-1 promoter was cloned in front of the daf-9 cDNA fused with gfp to generate array dhEx294 (14). Array dhEx24 contains the daf-9 cosmid T13C5 and pTG96 [sur-5::gfp]. muEx248 (Pdaf-16::gfp::daf-16) is an extrachromosomal array expressing daf-16::gfp under control of its own promoter as described in ref. 17.
Lifespan Experiments.
Δ4-dafachronic acid was synthesized in the Mangelsdorf Laboratory as described in ref. 11. To carry out aging experiments in the presence of ligand, Δ4-dafachronic acid was diluted in 100% ethanol mixed with the OP50 bacteria and pipetted onto 5-day-old plates 1 h before use at a 250 nM concentration. Every 3 days, worms were transferred to fresh plates containing ligand. As control, 100% ethanol was used. For dh6 and e1406 dauer recovery, partial dauer larvae were placed at 20°C. After 4–6 days, recovered larvae (those that had undergone radial expansion, vulval morphogenesis, formed adult alae, and initialized gametogenesis) were taken for further experiments at 15°C. Ten to 15 L4s were set up per nematode growth medium (NGM) agar plate. The number of dead versus live animals was determined every 2 to 3 days. Day 0 corresponds to the L4 stage. Experiments with daf-2 mutants were carried out at 22.5°C. For glp-1 germ-line agings, strains were cultured at 15°C for two generations. Parental generation 0 (P0) adults were allowed to lay eggs over a 4-h period to obtain a semisynchronous population, which were raised at 25°C until the L4 stage. These L4 larvae were put at 20°C or kept at 25°C for the lifespan assay. Unless noted otherwise, each experiment was repeated at least twice. P values were calculated on the pooled data of all of the experiments done in each set by using the log-rank (Mantel–Cox) method with the web-based interface of The Walter & Eliza Hall Institute of Medical Research (http://bioinf.wehi.edu.au/software/russell/logrank/index.html). P values <0.005 are considered significant.
Heat Stress and Oxidative Stress Experiments.
Worm strains were grown for two or more generations under replete conditions at 20°C. To induce heat stress, 1- or 3-day-old adults were transferred to 35°C, and survival was measured over a 12- to 30-h period. For oxidative stress assays, 1- or 3-day-old adults were transferred to plates containing 4.4–8.4 mM H2O2 (Sigma, St. Louis, MO), which was added onto the bacterial lawn 30 min before transfer of worms. Survival was scored at 1- to 2-h intervals over an 8- to 10-h period at 20°C. Experiments were repeated at least twice. P values were calculated by using the log-rank (Mantel–Cox) method on single experiments comparing strains examined at the same time.
DAF-16::GFP Nuclear Localization Experiments.
The strains carrying the muEx248 array were treated as described above for the glp germ-line agings. Semisynchronous eggs were transferred onto plates with Δ4-dafachronic acid or ethanol at 25°C. After 3 days, at day 1 of adulthood, worms were scored for nuclear localization of the DAF-16::GFP in the intestinal cells under a dissection scope (magnification of ×100). Worms with a clearly dotted appearance were scored as localized. Each experiment was performed at least two times. One experiment was performed blind and gave the same results.
Supplementary Material
Acknowledgments
We thank the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis, MN) and the Kenyon Laboratory (University of California, San Francisco, CA) for nematode strains and Scott Pletcher for comments on the manuscript. This work was generously supported by the Glenn/American Federation for Aging Research Breakthroughs in Gerontology Award (to A.A.), the National Institutes of Health (A.A. and D.J.M.), the Nuclear Receptor Signaling Atlas (A.A. and D.J.M.), the Howard Hughes Medical Institute (D.J.M.), the Robert Welch Foundation (D.J.M.), Max-Planck-Gesellschaft (A.A., B.G., and H.L.), and the National Genome Research Network 2 (B.G., H.L., and A.A.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700847104/DC1.
References
- 1.Kenyon C. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Larsen PL, Albert PS, Riddle DL. Genetics. 1995;139:1567–1583. doi: 10.1093/genetics/139.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gems D, Sutton AJ, Sundermeyer ML, Albert PS, King KV, Edgley ML, Larsen PL, Riddle DL. Genetics. 1998;150:129–155. doi: 10.1093/genetics/150.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsin H, Kenyon C. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 5.Jia K, Albert PS, Riddle DL. Development (Cambridge,UK) 2002;129:221–231. doi: 10.1242/dev.129.1.221. [DOI] [PubMed] [Google Scholar]
- 6.Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. Dev Cell. 2001;1:841–851. doi: 10.1016/s1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- 7.Simon AF, Shih C, Mack A, Benzer S. Science. 2003;299:1407–1410. doi: 10.1126/science.1080539. [DOI] [PubMed] [Google Scholar]
- 8.Antebi A, Yeh WH, Tait D, Hedgecock EM, Riddle DL. Genes Dev. 2000;14:1512–1527. [PMC free article] [PubMed] [Google Scholar]
- 9.Riddle DL, Swanson MM, Albert PS. Nature. 1981;290:668–671. doi: 10.1038/290668a0. [DOI] [PubMed] [Google Scholar]
- 10.Antebi A, Culotti JG, Hedgecock EM. Development (Cambridge, UK) 1998;125:1191–1205. doi: 10.1242/dev.125.7.1191. [DOI] [PubMed] [Google Scholar]
- 11.Motola DL, Cummins CL, Rottiers V, Sharma K, Sunino K, Xu E, Auchus R, Antebi A, Mangelsdorf M. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 12.Held JM, White MP, Fisher AL, Gibson BW, Lithgow GJ, Gill MS. Aging Cell. 2006;5:283–291. doi: 10.1111/j.1474-9726.2006.00218.x. [DOI] [PubMed] [Google Scholar]
- 13.Ohkura K, Suzuki N, Ishihara T, Katsura I. Development (Cambridge, UK) 2003;130:3237–3248. doi: 10.1242/dev.00540. [DOI] [PubMed] [Google Scholar]
- 14.Gerisch B, Antebi A. Development (Cambridge, UK) 2004;131:1765–1776. doi: 10.1242/dev.01068. [DOI] [PubMed] [Google Scholar]
- 15.Mak HY, Ruvkun G. Development (Cambridge, UK) 2004;131:1777–1786. doi: 10.1242/dev.01069. [DOI] [PubMed] [Google Scholar]
- 16.Apfeld J, Kenyon C. Cell. 1998;95:199–210. doi: 10.1016/s0092-8674(00)81751-1. [DOI] [PubMed] [Google Scholar]
- 17.Libina N, Berman JR, Kenyon C. Cell. 2003;115:489–502. doi: 10.1016/s0092-8674(03)00889-4. [DOI] [PubMed] [Google Scholar]
- 18.Wolkow CA, Kimura KD, Lee MS, Ruvkun G. Science. 2000;290:147–150. doi: 10.1126/science.290.5489.147. [DOI] [PubMed] [Google Scholar]
- 19.Larsen PL. Proc Natl Acad Sci USA. 1993;90:8905–8909. doi: 10.1073/pnas.90.19.8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lithgow GJ, White TM, Melov S, Johnson TE. Proc Natl Acad Sci USA. 1995;92:7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ludewig AH, Kober-Eisermann C, Weitzel C, Bethke A, Neubert K, Gerisch B, Hutter H, Antebi A. Genes Dev. 2004;18:2120–2133. doi: 10.1101/gad.312604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- 23.Rottiers V, Motola DL, Gerisch B, Cummins CL, Nishiwaki K, Mangelsdorf DJ, Antebi A. Dev Cell. 2006;10:473–482. doi: 10.1016/j.devcel.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Lin K, Hsin H, Libina N, Kenyon C. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 25.Berman JR, Kenyon C. Cell. 2006;124:1055–1068. doi: 10.1016/j.cell.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 26.Houten SM, Watanabe M, Auwerx J. EMBO J. 2006;25:1419–1425. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amador-Noguez D, Yagi K, Venable S, Darlington G. Aging Cell. 2004;3:423–441. doi: 10.1111/j.1474-9728.2004.00125.x. [DOI] [PubMed] [Google Scholar]
- 28.Russell DW. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 29.Song C, Liao S. Endocrinology. 2000;141:4180–4184. doi: 10.1210/endo.141.11.7772. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.