Abstract
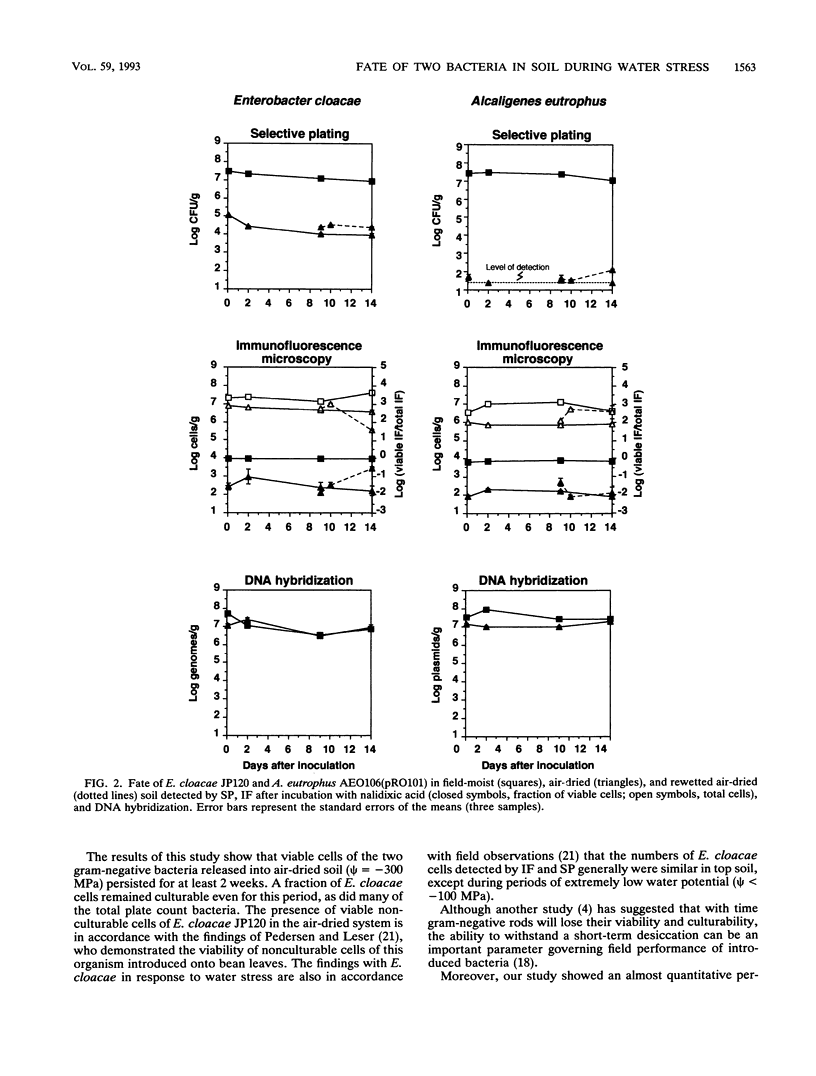
A sandy loam soil near field capacity moisture content (psi = -0.050 MPa) or air dried (psi = -300 MPa) was inoculated with about 3 x 10(7) CFU of Enterobacter cloacae JP120 and Alcaligenes eutrophus AEO106(pRO101) per g and incubated in 40-g portions at 17 degrees C in closed or open Erlenmeyer flasks. In the field-moist soil, selective plating, direct viable counts, and DNA hybridization showed only minor changes in the numbers of E. cloacae and A. eutrophus cells with time (14 days), and the results obtained with the three detection methods generally agreed. In the air-dried soil, the majority of both bacteria were found as intact DNA-carrying cells that were neither culturable nor viable by the methods employed in this study. The numbers of culturable E. cloacae and A. eutrophus cells dropped to 10(5) and 10(2) CFU/g, respectively, 2 h after inoculation. Direct viable counts showed that only about 1% of the cells detected by immunofluorescence microscopy were viable, but a fraction of viable nonculturable cells of both bacteria was present. A. eutrophus did not tolerate desiccation as well as E. cloacae. Only a minor fraction of the two test organisms regained their culturability or viability after rewetting of the air-dried soil; the number of total heterotrophic culturable bacteria, however, increased more than 10-fold and reached 73% of the level found in the field-moist soil at day 14.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottomley P. J., Dughri M. H. Population Size and Distribution of Rhizobium leguminosarum bv. trifolii in Relation to Total Soil Bacteria and Soil Depth. Appl Environ Microbiol. 1989 Apr;55(4):959–964. doi: 10.1128/aem.55.4.959-964.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker A. R., Olsen R. H., Seidler R. J. Phenoxyacetic acid degradation by the 2,4-dichlorophenoxyacetic acid (TFD) pathway of plasmid pJP4: mapping and characterization of the TFD regulatory gene, tfdR. J Bacteriol. 1989 Jan;171(1):314–320. doi: 10.1128/jb.171.1.314-320.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff K. A. Rapid and simple method for double staining of bacteria with 4',6-diamidino-2-phenylindole and fluorescein isothiocyanate-labeled antibodies. Appl Environ Microbiol. 1988 Dec;54(12):2949–2952. doi: 10.1128/aem.54.12.2949-2952.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A., Colwell R. R., Rahman R., Ali A., Chowdhury M. A., Parveen S., Sack D. A., Russek-Cohen E. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl Environ Microbiol. 1990 Aug;56(8):2370–2373. doi: 10.1128/aem.56.8.2370-2373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen C. S., Rasmussen O. F. Development and application of a new method to extract bacterial DNA from soil based on separation of bacteria from soil with cation-exchange resin. Appl Environ Microbiol. 1992 Aug;58(8):2458–2462. doi: 10.1128/aem.58.8.2458-2462.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary P., Ochin D., Tailliez R. Rates of Drying and Survival of Rhizobium meliloti Strains During Storage at Different Relative Humidities. Appl Environ Microbiol. 1985 Aug;50(2):207–211. doi: 10.1128/aem.50.2.207-211.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen J. C. Natamycin as a fungicide in agar media. Appl Environ Microbiol. 1992 Mar;58(3):1064–1066. doi: 10.1128/aem.58.3.1064-1066.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan R. J., Goksøyr J., Bej A. K., Atlas R. M. Recovery of DNA from soils and sediments. Appl Environ Microbiol. 1988 Dec;54(12):2908–2915. doi: 10.1128/aem.54.12.2908-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ley P., Bekkers A., Van Meersbergen J., Tommassen J. A comparative study on the phoE genes of three enterobacterial species. Implications for structure-function relationships in a pore-forming protein of the outer membrane. Eur J Biochem. 1987 Apr 15;164(2):469–475. doi: 10.1111/j.1432-1033.1987.tb11080.x. [DOI] [PubMed] [Google Scholar]