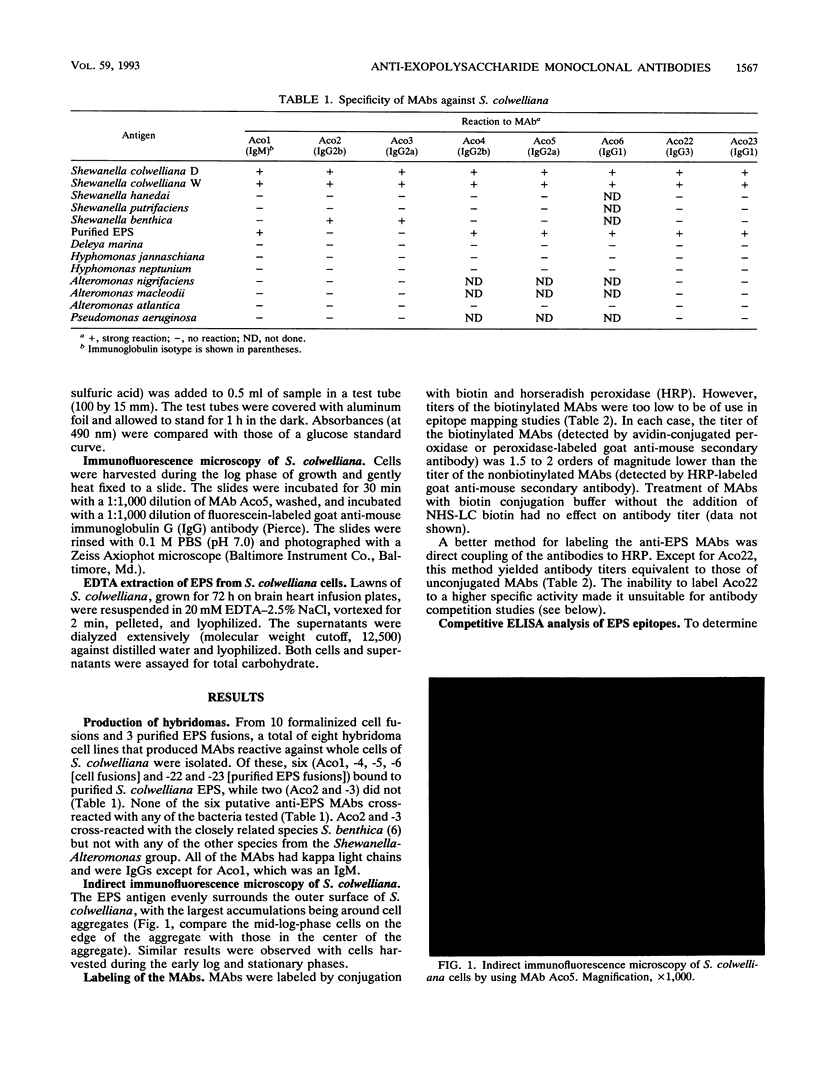
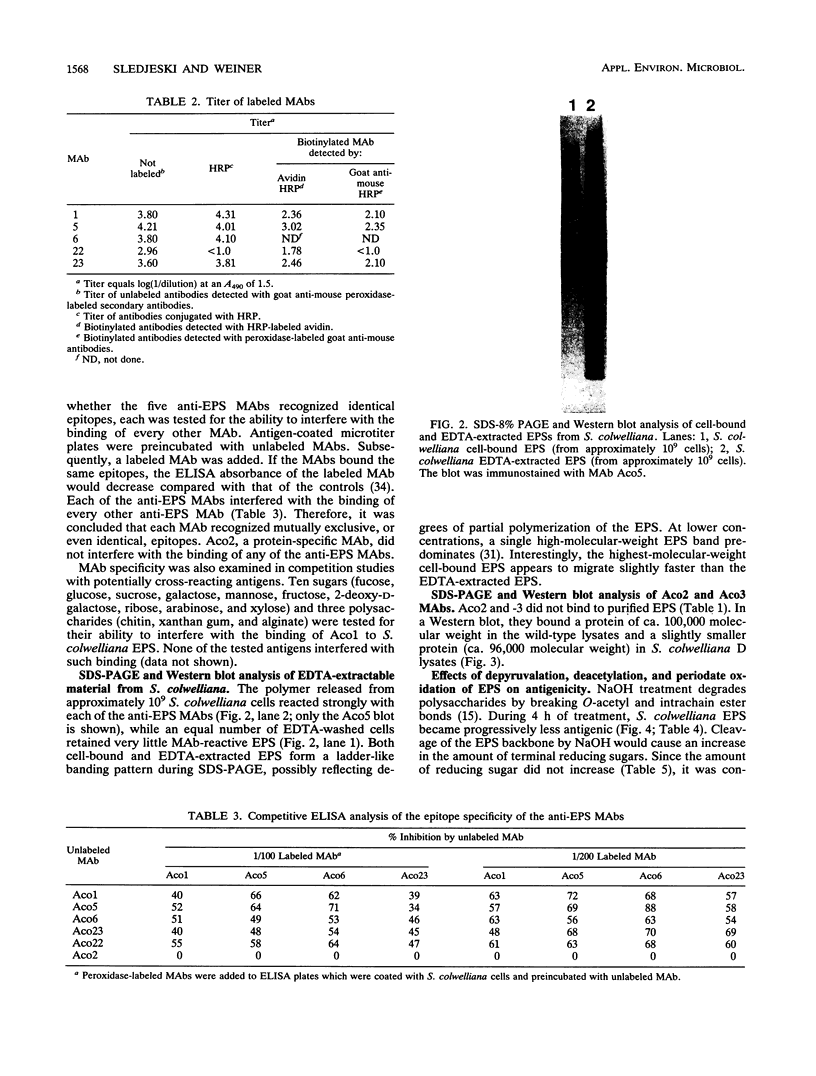
Abstract
Six monoclonal antibodies were produced to whole cells of Shewanella colwelliana (Aco1 to Aco6) and two (Aco22 to Aco23) to purified exopolysaccharide (EPS). Aco1, -4 to -6, -22, and -23 bound to both the cell surface and the purified EPS, while Aco2 and -3 bound to cells only. The EPS of S. colwelliana was antigenically unique from those of nine other species of marine bacteria that were tested. Mapping studies revealed that all of the EPS-specific monoclonal antibodies bound to the same epitope. This EPS epitope was sensitive to cleavage of ester bonds, but neither pyruvate, acetate, nor terminal nonreducing sugars were required for antigenicity. When S. colwelliana was grown on rich media, most of its EPS was loosely associated with the cell surface.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou C. E. A study of the immunochemistry of three yeast mannans. J Biol Chem. 1970 Mar 10;245(5):1197–1203. [PubMed] [Google Scholar]
- Bortolussi R., Ferrieri P., Björkstén B., Quie P. G. Capsular K1 polysaccharide of Escherichia coli: relationship to virulence in newborn rats and resistance to phagocytosis. Infect Immun. 1979 Jul;25(1):293–298. doi: 10.1128/iai.25.1.293-298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen B. E., Kjosbakken J., Smidsrød O. Partial Chemical and Physical Characterization of Two Extracellular Polysaccharides Produced by Marine, Periphytic Pseudomonas sp. Strain NCMB 2021. Appl Environ Microbiol. 1985 Oct;50(4):837–845. doi: 10.1128/aem.50.4.837-845.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Haaheim L. R., Kleppe G., Sutherland I. W. Monoclonal antibodies reacting with the exopolysaccharide xanthan from Xanthomonas campestris. J Gen Microbiol. 1989 Mar;135(3):605–612. doi: 10.1099/00221287-135-3-605. [DOI] [PubMed] [Google Scholar]
- Homonylo M. K., Wilmot S. J., Lam J. S., MacDonald L. A., Whitfield C. Monoclonal antibodies against the capsular K antigen of Escherichia coli (O9:K30(A):H12): characterisation and use in analysis of K antigen organisation on the cell surface. Can J Microbiol. 1988 Oct;34(10):1159–1165. doi: 10.1139/m88-204. [DOI] [PubMed] [Google Scholar]
- Hungerer D., Jann K., Jann B., Orskov F., Orskov I. Immunochemistry of K antigens of Escherichia coli. 4. The K antigen of E. coli O 9:K30:H12. Eur J Biochem. 1967 Jul;2(1):115–126. doi: 10.1111/j.1432-1033.1967.tb00115.x. [DOI] [PubMed] [Google Scholar]
- KABAT E. A., BEZER A. E. The effect of variation in molecular weight on the antigenicity of dextran in man. Arch Biochem Biophys. 1958 Dec;78(2):306–318. doi: 10.1016/0003-9861(58)90354-0. [DOI] [PubMed] [Google Scholar]
- KERBY G. P. A comparison of the removal of mucoid and non-mucoid variants of Klebsiella pneumoniae type B from the splanchnic circulating blood of the intact animal. J Immunol. 1950 Mar;64(3):131–137. [PubMed] [Google Scholar]
- Kabat E. A. The nature of an antigenic determinant. J Immunol. 1966 Jul;97(1):1–11. [PubMed] [Google Scholar]
- Lane R. D., Crissman R. S., Lachman M. F. Comparison of polyethylene glycols as fusogens for producing lymphocyte-myeloma hybrids. J Immunol Methods. 1984 Aug 3;72(1):71–76. doi: 10.1016/0022-1759(84)90434-4. [DOI] [PubMed] [Google Scholar]
- MacLEOD C. M., KRAUS M. R. Relation of virulence of pneumococcal strains for mice to the quantity of capsular polysaccharide formed in vitro. J Exp Med. 1950 Jul 1;92(1):1–9. doi: 10.1084/jem.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal S., Cross A., Gemski P. K antigen and serum sensitivity of rough Escherichia coli. Infect Immun. 1982 Sep;37(3):956–960. doi: 10.1128/iai.37.3.956-960.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke W. C., Ballou C. E. Characterization of a yeast mannan containing N-acetyl-D-glucosamine as an immunochemical determinant. Biochemistry. 1972 Sep 26;11(20):3807–3816. doi: 10.1021/bi00770a021. [DOI] [PubMed] [Google Scholar]
- Read R. R., Costerton J. W. Purification and characterization of adhesive exopolysaccharides from Pseudomonas putida and Pseudomonas fluorescens. Can J Microbiol. 1987 Dec;33(12):1080–1090. doi: 10.1139/m87-189. [DOI] [PubMed] [Google Scholar]
- Sledjeski D. Analysis of an acidic polysaccharide by electrophoretic transfer to nitrocellulose and immunostaining. Biotechniques. 1990 Oct;9(4):414–419. [PubMed] [Google Scholar]
- Smith H. W., Huggins M. B. The association of the O18, K1 and H7 antigens and the Co1V plasmid of a strain of Escherichia coli with its virulence and immunogenicity. J Gen Microbiol. 1980 Dec;121(2):387–400. doi: 10.1099/00221287-121-2-387. [DOI] [PubMed] [Google Scholar]
- Stähli C., Miggiano V., Stocker J., Staehelin T., Häring P., Takács B. Distinction of epitopes by monoclonal antibodies. Methods Enzymol. 1983;92:242–253. doi: 10.1016/0076-6879(83)92023-2. [DOI] [PubMed] [Google Scholar]
- Weiner R. M., Segall A. M., Colwell R. R. Characterization of a Marine Bacterium Associated with Crassostrea virginica (the Eastern Oyster). Appl Environ Microbiol. 1985 Jan;49(1):83–90. doi: 10.1128/aem.49.1.83-90.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein R., Young L. S. Phagocytic resistance of Escherichia coli K-1 isolates and relationship to virulence. J Clin Microbiol. 1978 Dec;8(6):748–755. doi: 10.1128/jcm.8.6.748-755.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. F., Smith A. L., Anderson P., Smith D. H. The role of encapsulation and host age in the clearance of Haemophilus influenzae bacteremia. J Infect Dis. 1977 Jan;135(1):34–41. doi: 10.1093/infdis/135.1.34. [DOI] [PubMed] [Google Scholar]
- Woodward M. P., Young W. W., Jr, Bloodgood R. A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985 Apr 8;78(1):143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- Wrangstadh M., Szewzyk U., Ostling J., Kjelleberg S. Starvation-specific formation of a peripheral exopolysaccharide by a marine Pseudomonas sp., strain S9. Appl Environ Microbiol. 1990 Jul;56(7):2065–2072. doi: 10.1128/aem.56.7.2065-2072.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]