Abstract
We describe a hypothalamus-specific mRNA that encodes preprohypocretin, the putative precursor of a pair of peptides that share substantial amino acid identities with the gut hormone secretin. The hypocretin (Hcrt) protein products are restricted to neuronal cell bodies of the dorsal and lateral hypothalamic areas. The fibers of these neurons are widespread throughout the posterior hypothalamus and project to multiple targets in other areas, including brainstem and thalamus. Hcrt immunoreactivity is associated with large granular vesicles at synapses. One of the Hcrt peptides was excitatory when applied to cultured, synaptically coupled hypothalamic neurons, but not hippocampal neurons. These observations suggest that the hypocretins function within the CNS as neurotransmitters.
The hypothalamus acts as a major regulatory center for autonomic and endocrine homeostasis. Structurally, it is a confederation of nuclei that regulate a broad array of physiological and behavioral activities. For some of these activities, particular peptides have been identified as major products of individual nuclei (1). These peptides exert their actions by transport to the pituitary, by entering the general circulation, or by secretion within the CNS. However, the hypothalamus has been implicated in the regulation of activities beyond those for which factors have been identified.
We recently used directional tag PCR subtraction to identify 38 rat mRNAs selectively expressed within the hypothalamus (2). Preliminary in situ hybridization studies revealed that one of these, called clone 35 in that work, was expressed exclusively by a bilaterally symmetric structure within the posterior hypothalamus. Here we show that the clone 35 mRNA encodes the precursor of two putative peptides, the hypocretins, that share substantial amino acid identities with each other and with the gut hormone secretin. The Hcrt mRNA, which accumulates primarily after postnatal week 3 and in mouse is a product of a gene on chromosome 11, is restricted to neuronal cell bodies of the dorsal and lateral hypothalamus. Its protein product, visualized immunocytochemically, is sorted into secretory vesicles in fibers that project within the hypothalamus and to other brain areas. At least one of the peptides has neuroexcitatory activity. Cumulatively, these observations suggest that the Hcrt mRNA encodes peptides that act endogenously within the central nervous system as homeostatic regulators. The circuitry revealed by the immunohistochemistry suggests a role in nutritional homeostasis.
MATERIALS AND METHODS
Production of Antisera.
Antiserum 2050 was generated by coupling the synthetic 17-mer CPTATATALAPRGGSRV to the carrier keyhole limpet hemocyanin with glutaraldehyde and immunizing rabbits as described (3). In Western transfer blots using as target electrophoretically separated proteins from bacteria transformed with the plasmid pRSET B engineered to express preprohcrt, we observed a single prominent immunoreactive band with a migration of ≈19 kDa with the hyperimmune serum, but not with the preimmune serum (not shown). No immunoreaction was detected with an extract from bacteria transformed with a preprocortistatin/pRSET B expression plasmid, indicating that detection of the 19-kDa target requires Hcrt expression. Analogous results were obtained with an additional antiserum to the 17 mer and two antisera to synthetic Hcrt2.
Immunohistochemistry.
Adult male Wistar rats were deeply anesthetized with Nembutal and perfused transcardially with 0.9% saline followed by 4% paraformaldehyde and 0.25% glutaraldehyde in 0.1 M phosphate buffer. The brains were postfixed for 12 h, cryoprotected with 30% sucrose for 48 h, and sliced into 20 μm-thick coronal sections. The sections were incubated in (i) antiserum 2050 (1:5,000) overnight; (ii) anti-rabbit biotinylated Ig (1:2,000) 90 min; (iii) avidin-biotin-peroxidase complex (1:1,000) 90 min. Labeling was then developed using diaminobenzidine-nickel. No staining was observed when the primary antibody was omitted or when the serum was preincubated for 24 h with the synthetic 17-mer (10 μg/ml) or with bacterially expressed protein. Neither Hcrt2 nor secretin blocked the immunoreactivity.
For immunoelectron microscopy, 60–80 μm cryoprotected coronal vibratome sections were incubated in 5% normal goat serum in PBS for 1 hour, rinsed twice in PBS for 5 min, incubated in the primary antiserum diluted 1:1,000–1:2,000 in 5% normal goat serum in PBS for 24–48 h at 4°C, and rinsed three times for 10 min with PBS. The primary antibody was detected by an avidin-biotin complex with horseradish peroxidase as the marker. Incubation times with the biotinylated secondary antibody and the avidin-biotin peroxidase complex were 12–24 h, and a solution of 0.05% diaminobenzidine hydrochloride in dilute phosphate buffer containing 0.003% hydrogen peroxide was used to develop the reaction. Sections were rinsed exhaustively in sodium phosphate buffer, exposed to 2% osmium tetroxide in phosphate buffer for 1 h, rinsed, then dehydrated through a graded series of ethanols, and flat embedded in TAAB resin. Sections cut on a Reichert Ultra Cut E microtome were mounted on 200-mesh copper grids, counterstained with uranyl acetate and lead citrate, and viewed in a Zeiss EM10 microscope.
RESULTS
Rat Preprohypocretin Contains Two Peptides with Related Sequences.
We used the original rat cDNA clone 35 to isolate full-length cDNAs for both rat and mouse. The 569 nucleotide rat sequence has the potential to encode a 130-residue putative secretory protein (preprohypocretin) with an apparent signal sequence and three additional sites for potential proteolytic maturation (Fig. 1A). Two of the four putative products of proteolysis, hypocretin 1 (residues 28–66) and hypocretin 2 (residues 69–97) have 14 amino acid identities across 20 residues (consensus sequence in Fig. 1B). This region of one of the peptides contains a 7/7 match with secretin (Fig. 1B), suggesting that the prepropeptide gives rise to two peptide products that are structurally related both to each other and to secretin.
Figure 1.
The hypocretins are novel, secretin-related peptides. (A) Hypocretin rat/mouse nucleotide (coding region only) and amino acid sequences. Differences in nucleotide sequences are indicated by asterisks below each different base, amino acid differences are indicated above triplets. Tandem basic amino acids (putative sites for proteolytic maturation) are indicated in bold, as is the serine residue most likely to represent the end of the secretion signal. (B) Alignment of Hcrt1 and Hcrt2 with rat secretin. The first nine residues of secretin have been repeated to indicate apparent circular permutation. The identities between the hypocretins and members of the glucagon/vasoactive intestinal polypeptide/secretin family (3) are indicated by asterisks, the Hcrt1/2 consensus residues appear above the alignment. The complete rat and mouse sequences were obtained by standard methods.
The identity between the Hcrt peptides and secretin is not preserved with the several other members of the glucagon/vasoactive intestinal polypeptide/secretin (incretin) peptide family (4). However, alignments (not shown) of the sequences of the two new peptides with other family members reveal additional sequence identities between N-terminal residues in the family members and C-terminal residues of the Hcrts, suggesting that the hypocretins represent members of this family whose primary amino acid sequences are circularly permuted (the secretin sequence in Fig. 1B is partially repeated head to tail to show this feature).
Two Secretin-Related Peptides Conserved with Mouse.
The mouse Hcrt nucleotide sequence differs in 39 positions relative to the rat, and contains 13 additional nucleotides within its 3′ untranslated region. Of these differences, 19 are within the putative protein-coding region (Fig. 1A), only seven of which affect the encoded protein sequence: one amino acid difference at residue 3 is a neutral substitution in the apparent secretion signal sequence; the remaining six differences are near the C terminus, one of which obliterates a potential proteolytic cleavage site. The absence of this site and the nature of the other differences make it unlikely that two of the four possible rat maturation products are generated and functional in mice. However, Hcrt1 and Hcrt2 are absolutely preserved between rat and mouse, providing strong support for the notion that an important functional signal has been conserved during evolution.
Both Hcrts terminate with glycine residues, which typically are substrates for peptidylglycine alpha-amidating monooxygenase, leaving a C-terminal amide in the mature peptide (5). The N-terminal extent of Hcrt1 is not established.
Mouse Gene on Chromosome 11.
Using an interspecific backcross, we mapped the mouse Hcrt gene to chromosome 11, a region that shows conserved synteny with human chromosome 17q21-q24. A single-strand sequence polymorphism between C57BL/6J and SPRET/Ei was detected as described (6) and mapped on The Jackson Laboratory BSS panel. An hcrt-specific product of approximately 600 base pairs was amplified from mouse C57BL/6J genomic DNA using synthetic oligonucleotides 5′-GACGGCCTCAGACTTCTTGG-3′ and 5′-GCAACAGTTCGTAGAGACGG-3′. This product contained a putative intron, and its identity as hcrt was confirmed by sequencing. No recombinants in 94 BSS mice were found between Hcrt and the previously mapped loci Brca1, Tubg, and Mpmv8, placing Hcrt maximally within 3.8 centimorgans (cM) (95% confidence limit) of these genes. The Hoxb cluster is ≈1 cM centromeric to Hcrt, and the Kcnj2 gene is located ≈4 cM telomeric. Genotype data and references for these and other linked markers can be accessed via the Mouse Genome Database (http://www.informatics.jax.org).
RNA Is Restricted to Hypothalamus and Accumulates After the Third Postnatal Week.
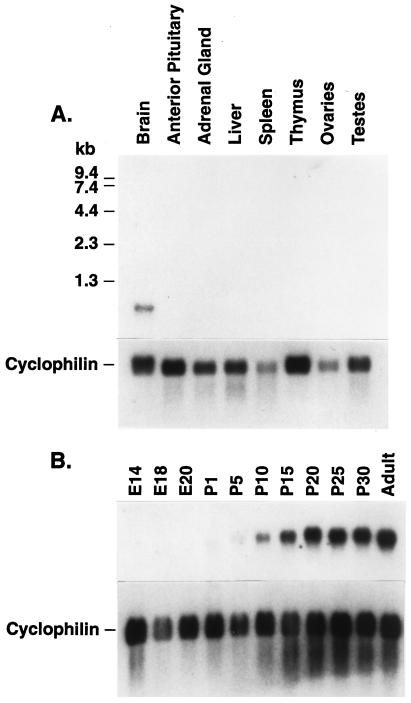
In Northern blot studies using poly(A)+ RNA prepared from brain and different peripheral tissues, we detected the 700-nucleotide Hcrt mRNA only in brain samples (Fig. 2A). Previous studies with RNA from different regions of the brain had detected the Hcrt mRNA predominantly in hypothalamus samples (2). In samples of RNA from whole brains of developing rats, Hcrt mRNA was detected at low concentrations as early as embryonic day 18, but increased in concentration dramatically after the third postnatal week (Fig. 2B). There was no detectable difference between brain samples from adult males and females (not shown), suggesting that Hcrt function is not related to sexually dimorphic processes. In situ hybridization studies detected cell bodies in the dorsal-lateral hypothalamus (Fig. 3A).
Figure 2.
Hypocretin accumulates postnatally specifically in the brain. Northern blot, prepared by standard methods with poly(A)+ from the indicated tissues of (A) adult rats or (B) whole brains (E14 was whole head) from developing rats of the indicated embryonic (E) or postnatal (P) ages. In each case the blot was stripped and reprobed with a cDNA for the ubiquitously expressed cyclophilin as a loading and RNA integrity control.
Figure 3.
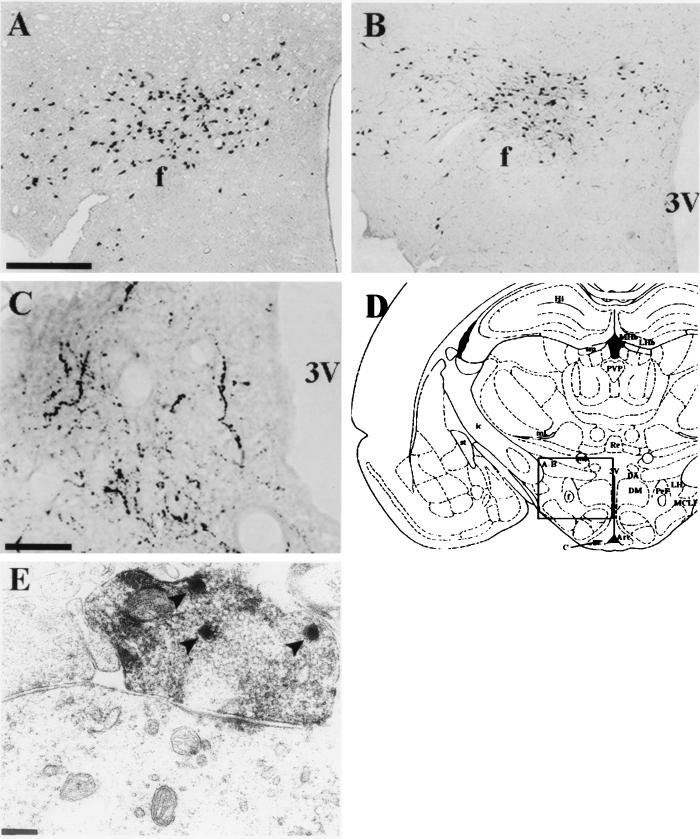
Hypocretin is expressed in a unique pattern within the hypothalamus. (A) In situ hybridization, performed on free-floating sections as described (40), detects cells in the posterior hypothalamus. Bar = 250 μm (A and B). (B) Immunohistochemical localization of cells containing the preprohypocretin peptide at a rostral-caudal level of the hypothalamus comparable to the view in A. Positive neurons in A and B appear in three clusters: the perifornical, dorsal and lateral hypothalamic areas. Immunoreactivity was observed at levels 25–27 (see ref. 7), and about plates 28–31 of ref. 8. (C) Immunoreactive fibers within the arcuate nucleus of the hypothalamus. Abbreviations: f, fornix; 3V, third ventricle. Bar = 50 μm. (D) Schematic drawing of a coronal section of the rat brain (from ref. 8) at the level shown in A and B where most hyponcretin-positive cell bodies are located. Greater than 80% of the mRNA-positive and immunoreactive cell bodies are enclosed by the area defined by the square and extend at least 1 mm rostral-caudal from this coronal plane. The area shown in C is indicated by the arrow, however the plane of section was more caudal than the drawing. (E) Electron micrograph of immunoreactive bouton synapsing on dendritic shaft in the periaqueductal gray of the thalamus. Arrowheads indicate the electron-dense peroxidase staining of large, dense-core vesicles. (Bar = 0.25 μm. Magnification, ×37,800.)
Detection of the Hypocretin Protein in Neuronal Cell Bodies and Fibers.
We raised a polyclonal antiserum (serum 2050) against a chemically synthesized peptide corresponding to the C-terminal 17 amino acids of the rat preprohcrt sequence. Immunohistochemical studies with antiserum 2050 on sections from perfused adult male rats, showed prominently granular immunoreactivity within widely spaced, large polymorphic (20–30 μm) neurons exclusively in the dorsal lateral hypothalamic area (Fig. 3B), coincident with the in situ hybridization-positive cells. This coincident staining, its elimination when the serum was preincubated with the peptide immunogen (not shown), and the very low nonspecific background observed, together with the Western blot studies described in Materials and Methods, provided strong evidence for the specificity of the antiserum for Hcrt. Analogous results were obtained with an antiserum (no. 2123) against bacterially expressed, histidine-tagged preprohcrt (not shown).
The reactive cell bodies were observed between the fornix and the mammillothalamic tracts, 1 mm lateral to the midline, at the level of the median eminence (7, 8). The Hcrt neurons span the perifornical nucleus and the magnocellular nucleus of the lateral hypothalamus from the medial hypothalamus across the suprafornical region at mid-to-posterior hypothalamic levels into the myelinated axons of the retrochiasmic optic radiation.
In addition to the hypothalamic neurons, antiserum 2050 and antiserum 2123 both detected a prominent network of axons located within the posterior hypothalamus (Fig. 3C shows a view of the arcuate nucleus) and beyond. A large number of fiber projections were observed in apparent terminal fields within septal nuclei in the basal forebrain, the preoptic area, the paraventricular nucleus of the thalamus, the central gray, and the locus coeruleus. Less prominent fiber projections were observed in apparent terminal fields within the colliculi, the laterodorsal tegmental nucleus, and the nucleus of the solitary tract.
Hypocretin in Synaptic Vesicles.
In electron microscopy studies, immunoreactivity was observed within the lateral hypothalamus on perikaryal rough endoplasmic reticulum, cytoplasmic large granular (800–1000 Å) vesicles as well as similar structures in dendrites (often postsynaptic to nonimmunoreactive terminals), and vesicles within myelinated axons and at presynaptic terminals opposite nonimmunoreactive dendrites. Within the relatively dense terminal fields in the periaqueductal gray, immunoreactive boutons consistently made asymmetrical synaptic contacts with small-to-medium-sized dendritic shafts (Fig. 3E). Boutons contained two to eight large, intensely immunoreactive granular vesicles in the plane of section, but in heavily reactive boutons a lighter peroxidase reaction was observed between the small agranular vesicles. The postsynaptic thickenings were usually single and occupied no more than one-third of the contact surfaces between the immunoreactive bouton and its dendrite.
Hcrt2 Is Neuroexcitatory.
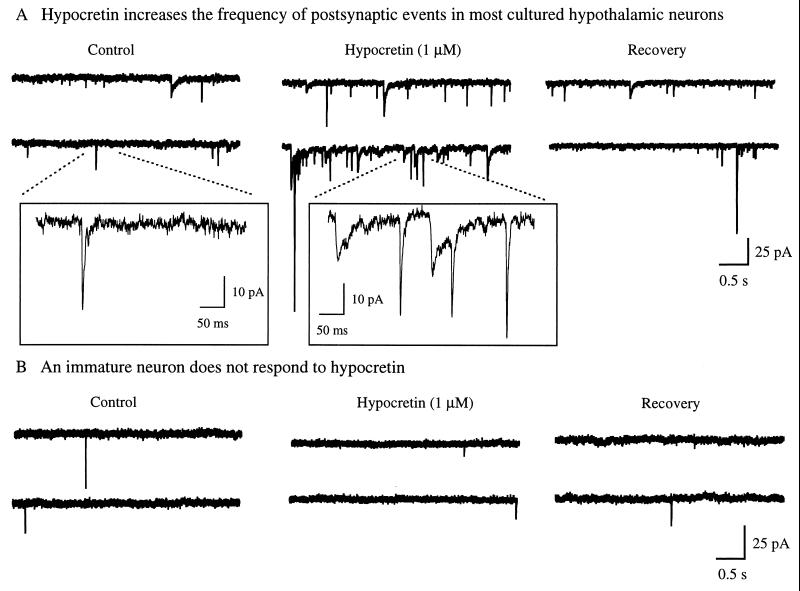
The putative structures of the hypocretins, their expression within the dorsolateral hypothalamus and accumulation within axon terminals and vesicles suggested that they may have intercellular signaling activity. To test this hypothesis, we studied 10-day-old cultures of synaptically coupled rat hypothalamic neurons and recorded postsynaptic currents under voltage clamp. Application of a synthetic peptide corresponding to amidated Hcrt2 (residues 69- to 96-amide) at 1 μM evoked a substantial, but reversible, increase in the frequency of postsynaptic currents in 75% of the neurons tested in six cultures (Fig. 4A), indicating an increase in the activity of axon terminals, and suggesting an increase in excitation. The other 25% of the cells showed no response to Hcrt2. There was little response by hypothalamic neurons that had been in culture for only 3–5 days, suggesting that a certain degree of synaptic maturity was required for the effect. Hcrt2 elicited no response in four cultures of synaptically coupled hippocampal dentate granule neurons (cells that do not express hcrt in vivo and where immunoreactive axons are rare), demonstrating target selectivity and suggesting that specific receptors for Hcrt2 may exist.
Figure 4.
Hypocretin 2 has biological activity. (A) Rat hypothalamus from 18-day embryos was cultured for 10 days in vitro. Synaptically coupled hypothalamic neurons were recorded in voltage clamp with a whole cell pipette (holding potential = −60 mV). This recording is typical of nine of 12 cells examined under these conditions. The frequency of postsynaptic events was greatly increased (up to +400%) by 1 μM Hcrt2 applied to the bath. After washout of the peptide, the frequency of postsynaptic events returned to normal baseline levels. (Inset) Higher resolution of the events are indicated by the dotted line. Both boxes (in A and B) were recorded with an identical delay after Hcrt2 administration. (B) A hypothalamic neuron after 4 days of culture was unresponsive to 1 μM Hcrt2. Pipette solution contained 128 mM KMeSO4, 27 mM KCl, 0.4 mM EGTA, 1 mM ATP, and 0.5 mM GTP.
DISCUSSION
Although establishment of an endogenous compound as a bona fide neurotransmitter or hormone requires rigorously meeting several criteria, this first characterization of the hypocretins, particularly Hcrt2, suggests that they should be considered as candidate peptide neurotransmitters. Their mRNA is expressed primarily after postnatal week 3 in a group of hypothalamic neurons not previously recognized to be biochemically related. The mRNA is translated to produce an immunoreactive product that is associated with vesicles, many of which are located at presynaptic structures. Cross-species comparisons suggest that Hcrt1 and Hcrt2 are the conserved products of the mRNA, and these putative, presumably secreted, peptides exhibit striking amino acid sequence identity with members of a family of known hormones, the incretins. Our name, hypocretin, is intended to indicate a hypothalamic member of the incretin family.
Synthetic Hcrt2 has excitatory activity selectively on hypothalamic cells in culture. By analogy with other members of the incretin family, these peptides may fold into structures that resemble the structures of secretin and its homologs, even though their primary sequences are permuted, and act through dedicated G protein-coupled receptors that activate adenylyl cyclase (9). Two Hcrt peptides that differ in their amino acid sequences might indicate two Hcrt receptor subtypes.
What organismic functions could the Hcrt neuropeptides subserve? Stereotactic ablation and physiological studies have previously implicated the dorsal-lateral hypothalamus in feeding behavior (10–13), blood pressure (14, 15), and central regulation of immune function (16–18), although precise nuclei have not been correlated with these activities. The projections of Hcrt-producing cells indicate that the peptides function both within the hypothalamus and at a complex and diffuse network of targets in several regions of the brain.
Several neuropeptides with cell bodies located in the hypothalamus have been implicated in the control of energy balance (19–32), both as promoters of food consumption (neuropeptide Y, galanin, and melanin-concentrating hormone), and as substances with anorectic or satiety-inducing effects (cholecystokinin, bombesin, corticotropin-releasing factor). The location of Hcrt-containing cell bodies in the posterior hypothalamus completely overlaps with that of melanin-concentrating hormone, although melanin-concentrating hormone has a more extensive distribution (33–36), suggesting the possibility that Hcrt is synthesized in some of the same neurons that also produce melanin-concentrating hormone, a peptide with potent orexigenic activity. Future work will address this hypothesis as well as determine the central nervous system locations at which the Hcrt peptides bind. In this regard, the endogenous agonist for the melanocortin-4 receptor (MC4-R), a receptor with widespread distribution in the central nervous system (37) similar to the distribution of Hcrt fibers, is not known. Inactivation of the MC4-R by gene targeting results in an obese phenotype associated with hyperphagia, hyperinsulinemia, and hyperglycemia (38), a phenotype at least partially mimicked by transgenic overexpression of the recently described Agouti-related protein AGRP, which acts in vitro as an MC4-R antagonist (39). Perhaps among their activities the Hcrt neuropeptides act as agonists at MC4-Rs in opposition to AGRP.
Acknowledgments
We thank Laurie McPherson, Devin Tighe and Briant Lee for excellent technical assistance. Supported in part by grants from the National Institutes of Health (GM32355, NS33396, AG11084), Air Force Office of Scientific Research (F49620-95-1-0247), Army Research Office (DAAH04-95-1-0616), Norwegian Research Council, Fyssen Foundation, and Digital Gene Technologies.
ABBREVIATION
- Hcrt
hypocretin
Footnotes
References
- 1.Swanson L W. In: Handbook of Chemical Anatomy. Bjorkland A, Hokfelt T, Swanson L W, editors. Vol. 5. New York: Elsevier; 1987. pp. 1–124. [Google Scholar]
- 2.Gautvik K M, de Lecea L, Gautvik V T, Danielson P E, Tranque P, Dopazo A, Bloom F E, Sutcliffe J G. Proc Natl Acad Sci USA. 1996;93:8733–8738. doi: 10.1073/pnas.93.16.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutcliffe J G, Milner R J, Shinnick T S, Bloom F E. Cell. 1983;33:671–682. doi: 10.1016/0092-8674(83)90010-7. [DOI] [PubMed] [Google Scholar]
- 4.Fehmann H-C, Goke R, Goke B. Endocr Rev. 1995;16:390–410. doi: 10.1210/edrv-16-3-390. [DOI] [PubMed] [Google Scholar]
- 5.Eipper B A, Stoffers D A, Mains R E. Annu Rev Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- 6.de Lecea L, Ruiz-Lozano P, Danielson P E, Peelle-Kirley J, Foye P E, Frankel W N, Sutcliffe J G. Genomics. 1997;42:499–506. doi: 10.1006/geno.1997.4763. [DOI] [PubMed] [Google Scholar]
- 7.Swanson L W. Brain Maps: Structure of the Rat Brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- 8.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 9.Segre G V, Goldring S R. Trends Endocrinol Metab. 1993;4:309–314. doi: 10.1016/1043-2760(93)90071-l. [DOI] [PubMed] [Google Scholar]
- 10.Bray G A, York D A. Physiol Rev. 1979;59:719–809. doi: 10.1152/physrev.1979.59.3.719. [DOI] [PubMed] [Google Scholar]
- 11.Teitelbaum L A, Epstein A N. Psychol Rev. 1960;69:74–90. doi: 10.1037/h0039285. [DOI] [PubMed] [Google Scholar]
- 12.Grossman S P, Dacey D, Halaris A E, Routenberg A. Science. 1978;202:537–539. doi: 10.1126/science.705344. [DOI] [PubMed] [Google Scholar]
- 13.Stricker E M, Verbalis J G. Nutr Rev. 1990;48:49–56. doi: 10.1111/j.1753-4887.1990.tb02905.x. [DOI] [PubMed] [Google Scholar]
- 14.Loewy A D, Spyer K M, editors. Central Regulation of Autonomic Functions. New York: Oxford Univ. Press; 1990. [Google Scholar]
- 15.Randall W C, editor. Nervous Control of Cardiovascular Function. New York: Oxford Univ. Press; 1984. [Google Scholar]
- 16.Berkenbosch F, van Oers J, del Ray A, Tilders F, Besedovsky H. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham E T, De Souza E B. Immunol Today. 1993;14:171–176. doi: 10.1016/0167-5699(93)90281-o. [DOI] [PubMed] [Google Scholar]
- 18.Rivier C. Front Neuroendocrinol. 1995;16:151–182. doi: 10.1006/frne.1995.1005. [DOI] [PubMed] [Google Scholar]
- 19.Morley J E. Endocr Rev. 1987;8:256–287. doi: 10.1210/edrv-8-3-256. [DOI] [PubMed] [Google Scholar]
- 20.Clark J T, Kalra P S, Crowley W R, Kalra S P. Endocrinology. 1984;115:427–429. doi: 10.1210/endo-115-1-427. [DOI] [PubMed] [Google Scholar]
- 21.Levine A S, Morley J E. Peptides (Tarrytown, NY) 1984;5:1025–1029. doi: 10.1016/0196-9781(84)90165-7. [DOI] [PubMed] [Google Scholar]
- 22.Stanley B G, Leibowitz S F. Life Sci. 1984;35:2635–2642. doi: 10.1016/0024-3205(84)90032-8. [DOI] [PubMed] [Google Scholar]
- 23.Kyrkouli S E, Stanley B G, Leibowitz S F. Eur J Pharmacol. 1986;122:159–160. doi: 10.1016/0014-2999(86)90175-5. [DOI] [PubMed] [Google Scholar]
- 24.Tempel D L, Leibowitz S F. Brain Res Bull. 1990;25:821–825. doi: 10.1016/0361-9230(90)90177-2. [DOI] [PubMed] [Google Scholar]
- 25.Qu D, Ludwig D S, Gammeltoft S, Piper M, Pelleymounter M A, Cullen M J, Mathes W F, Przypek J, Kanarek R, Maratos-Flier E. Nature (London) 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 26.Rossi M, Choi S J, O’Shea D, Miyoshi T, Ghatei M A, Bloom S R. Endocrinology. 1997;138:351–355. doi: 10.1210/endo.138.1.4887. [DOI] [PubMed] [Google Scholar]
- 27.Smith G P, Gibbs J. Pharmacol Biochem Behav. 1975;3:135–138. [PubMed] [Google Scholar]
- 28.Gibbs J, Fauser D J, Rowe E A, Rolls B J, Rolls E T, Madison S P. Nature (London) 1979;282:208–210. doi: 10.1038/282208a0. [DOI] [PubMed] [Google Scholar]
- 29.Britton D R, Koob G F, Rivier J, Vale W. Life Sci. 1982;31:363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- 30.Levine A S, Rogers B, Kneip J, Grace M, Morley J E. Neuropharmacology. 1983;22:337–339. doi: 10.1016/0028-3908(83)90249-6. [DOI] [PubMed] [Google Scholar]
- 31.Arase K, York D A, Shimizu H, Shargill N, Bray G A. Am J Physiol. 1988;255:E255–E259. doi: 10.1152/ajpendo.1988.255.3.E255. [DOI] [PubMed] [Google Scholar]
- 32.Spina M, Merlo-Pich E, Chan R K W, Basso A M, Rivier J, Vale W, Koob G F. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- 33.Zamir N, Skofitsch G, Jacobowitz D M. Brain Res. 1986;373:240–245. doi: 10.1016/0006-8993(86)90337-9. [DOI] [PubMed] [Google Scholar]
- 34.Skofitsch G, Jacobowitz D M, Zamir N. Brain Res Bull. 1985;15:635–649. doi: 10.1016/0361-9230(85)90213-8. [DOI] [PubMed] [Google Scholar]
- 35.Naito N, Kawazoe I, Nakai Y, Kawauchi H. Cell Tissue Res. 1988;253:291–295. doi: 10.1007/BF00222284. [DOI] [PubMed] [Google Scholar]
- 36.Bittencourt J C, Presse F, Arias C, Peto C, Vaughan J, Nahon J L, Vale W, Sawchenko P E. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- 37.Mountjoy K G, Mortrud M T, Low M J, Simerly R B, Cone R D. Mol Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- 38.Huszar D, Lynch C A, Fairchild-Huntress V, Dunmore J H, Fang Q, Berkemeier L R, Gu W, Kesterson R A, Boston B A, Cone R D, Smith F J, Campfield L A, Burn P, Lee F. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 39.Ollmann M M, Wilson B D, Yang Y K, Kerns J A, Chen Y, Gantz I, Barsh G S. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 40.de Lecea L, Soriano E, Criado J R, Steffensen S C, Henriksen S J, Sutcliffe J G. Brain Res Mol Brain Res. 1994;25:286–296. doi: 10.1016/0169-328x(94)90164-3. [DOI] [PubMed] [Google Scholar]