Abstract
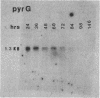
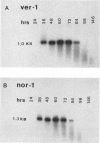
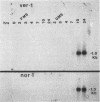
RNA transcript accumulation for the ver-1 and nor-1 genes, which are associated with aflatoxin biosynthesis in the fungus Aspergillus parasiticus, was measured before and during aflatoxin production in liquid shake culture. Transcripts were not detected until near the end of trophophase (growth phase) and could still be observed well into stationary phase during batch fermentation in an aflatoxin-supporting growth medium. Maximum accumulation of both transcripts occurred just prior to the onset of stationary phase. Aflatoxin B1 was first detected approximately 8 h after the appearance of the ver-1 and nor-1 transcripts. In contrast, maximum transcript accumulation for the pyrG gene (encoding orotidine monophosphate decarboxylase), which is involved in primary metabolism, was observed at the onset of trophophase when the ver-1 and nor-1 transcripts could not be detected. Accumulation of the ver-1 and nor-1 transcripts was also studied following a nutritional shift from a non-aflatoxin-supporting medium (peptone mineral salts [PMS]) to a glucose-containing medium (glucose mineral salts [GMS]), which does support aflatoxin biosynthesis. Transcripts from ver-1 and nor-1 could not be detected on PMS medium but did accumulate approximately 4 to 7 h following transfer to GMS medium. Additionally, aflatoxins were not detected in PMS medium but were observed to accumulate within 24 h after the shift from PMS to GMS. These data suggest that aflatoxin biosynthesis is in part regulated by the accumulation of the ver-1 and nor-1 transcripts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADYE J., MATELES R. I. INCORPORATION OF LABELLED COMPOUNDS INTO AFLATOXINS. Biochim Biophys Acta. 1964 May 11;86:418–420. doi: 10.1016/0304-4165(64)90077-7. [DOI] [PubMed] [Google Scholar]
- Bhatnagar D., Cleveland T. E., Kingston D. G. Enzymological evidence for separate pathways for aflatoxin B1 and B2 biosynthesis. Biochemistry. 1991 Apr 30;30(17):4343–4350. doi: 10.1021/bi00231a033. [DOI] [PubMed] [Google Scholar]
- Bhatnagar R. K., Ahmad S., Mukerji K. G., Venkitasubramanian T. A. Nitrogen metabolism in Aspergillus parasiticus NRRL 3240 and A. flavus NRRL 3537 in relation to aflatoxin production. J Appl Bacteriol. 1986 Mar;60(3):203–211. doi: 10.1111/j.1365-2672.1986.tb01074.x. [DOI] [PubMed] [Google Scholar]
- Buchanan R. L., Jones S. B., Gerasimowicz W. V., Zaika L. L., Stahl H. G., Ocker L. A. Regulation of aflatoxin biosynthesis: assessment of the role of cellular energy status as a regulator of the induction of aflatoxin production. Appl Environ Microbiol. 1987 Jun;53(6):1224–1231. doi: 10.1128/aem.53.6.1224-1231.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan R. L., Lewis D. F. Regulation of aflatoxin biosynthesis: effect of glucose on activities of various glycolytic enzymes. Appl Environ Microbiol. 1984 Aug;48(2):306–310. doi: 10.1128/aem.48.2.306-310.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang P. K., Skory C. D., Linz J. E. Cloning of a gene associated with aflatoxin B1 biosynthesis in Aspergillus parasiticus. Curr Genet. 1992 Mar;21(3):231–233. doi: 10.1007/BF00336846. [DOI] [PubMed] [Google Scholar]
- Chu F. S., Hsia M. T., Sun P. S. Preparation and characterization of aflatox-n B1-1-(O-carboxymethyl) oxime. J Assoc Off Anal Chem. 1977 Jul;60(4):791–794. [PubMed] [Google Scholar]
- Chu F. S. Mycotoxins: food contamination, mechanism, carcinogenic potential and preventive measures. Mutat Res. 1991 Mar-Apr;259(3-4):291–306. doi: 10.1016/0165-1218(91)90124-5. [DOI] [PubMed] [Google Scholar]
- Chuturgoon A. A., Dutton M. F., Berry R. K. The preparation of an enzyme associated with aflatoxin biosynthesis by affinity chromatography. Biochem Biophys Res Commun. 1990 Jan 15;166(1):38–42. doi: 10.1016/0006-291x(90)91908-b. [DOI] [PubMed] [Google Scholar]
- Cleveland T. E., Bhatnagar D. Evidence for de novo synthesis of an aflatoxin pathway methyltransferase near the cessation of active growth and the onset of aflatoxin biosynthesis in Aspergillus parasiticus mycelia. Can J Microbiol. 1990 Jan;36(1):1–5. doi: 10.1139/m90-001. [DOI] [PubMed] [Google Scholar]
- Cleveland T. E. Conversion of dihydro-O-methylsterigmatocystin to aflatoxin B2 by Aspergillus parasiticus. Arch Environ Contam Toxicol. 1989 May-Jun;18(3):429–433. doi: 10.1007/BF01062369. [DOI] [PubMed] [Google Scholar]
- Cleveland T. E., Lax A. R., Lee L. S., Bhatnagar D. Appearance of enzyme activities catalyzing conversion of sterigmatocystin to aflatoxin B1 in late-growth-phase Aspergillus parasiticus cultures. Appl Environ Microbiol. 1987 Jul;53(7):1711–1713. doi: 10.1128/aem.53.7.1711-1713.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew S. W., Demain A. L. Effect of primary metabolites on secondary metabolism. Annu Rev Microbiol. 1977;31:343–356. doi: 10.1146/annurev.mi.31.100177.002015. [DOI] [PubMed] [Google Scholar]
- Dutton M. F. Enzymes and aflatoxin biosynthesis. Microbiol Rev. 1988 Jun;52(2):274–295. doi: 10.1128/mr.52.2.274-295.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh D. P., Wan C. C., Billington J. A. A versiconal hemiacetal acetate converting enzyme in aflatoxin biosynthesis. Mycopathologia. 1989 Sep;107(2-3):121–126. doi: 10.1007/BF00707548. [DOI] [PubMed] [Google Scholar]
- Jelinek C. F., Pohland A. E., Wood G. E. Worldwide occurrence of mycotoxins in foods and feeds--an update. J Assoc Off Anal Chem. 1989 Mar-Apr;72(2):223–230. [PubMed] [Google Scholar]
- Lin B. K., Anderson J. A. Purification and properties of versiconal cyclase from Aspergillus parasiticus. Arch Biochem Biophys. 1992 Feb 14;293(1):67–70. doi: 10.1016/0003-9861(92)90366-5. [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Ayres J. C., Koehler P. E. Influence of temperature cycling on the production of aflatoxins B1 and G1 by Aspergillus parasiticus. Appl Environ Microbiol. 1980 Aug;40(2):333–336. doi: 10.1128/aem.40.2.333-336.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehaus W. G., Jr, Jiang W. P. Nitrate induces enzymes of the mannitol cycle and suppresses versicolorin synthesis in Aspergillus parasiticus. Mycopathologia. 1989 Sep;107(2-3):131–137. doi: 10.1007/BF00707550. [DOI] [PubMed] [Google Scholar]
- Pestka J. J. Enhanced surveillance of foodborne mycotoxins by immunochemical assay. J Assoc Off Anal Chem. 1988 Nov-Dec;71(6):1075–1081. [PubMed] [Google Scholar]
- Reddy T. V., Viswanathan L., Venkitasubramanian T. A. High aflatoxin production on a chemically defined medium. Appl Microbiol. 1971 Sep;22(3):393–396. doi: 10.1128/am.22.3.393-396.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Behere A. G., Padwal-Desai S. R., Nadkarni G. B. Influence of inoculum size of Aspergillus parasiticus spores on aflatoxin production. Appl Environ Microbiol. 1980 Dec;40(6):989–993. doi: 10.1128/aem.40.6.989-993.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Chang P. K., Cary J., Linz J. E. Isolation and characterization of a gene from Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl Environ Microbiol. 1992 Nov;58(11):3527–3537. doi: 10.1128/aem.58.11.3527-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skory C. D., Horng J. S., Pestka J. J., Linz J. E. Transformation of Aspergillus parasiticus with a homologous gene (pyrG) involved in pyrimidine biosynthesis. Appl Environ Microbiol. 1990 Nov;56(11):3315–3320. doi: 10.1128/aem.56.11.3315-3320.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabe K., Ando Y., Hashimoto J., Hamasaki T. Two distinct O-methyltransferases in aflatoxin biosynthesis. Appl Environ Microbiol. 1989 Sep;55(9):2172–2177. doi: 10.1128/aem.55.9.2172-2177.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
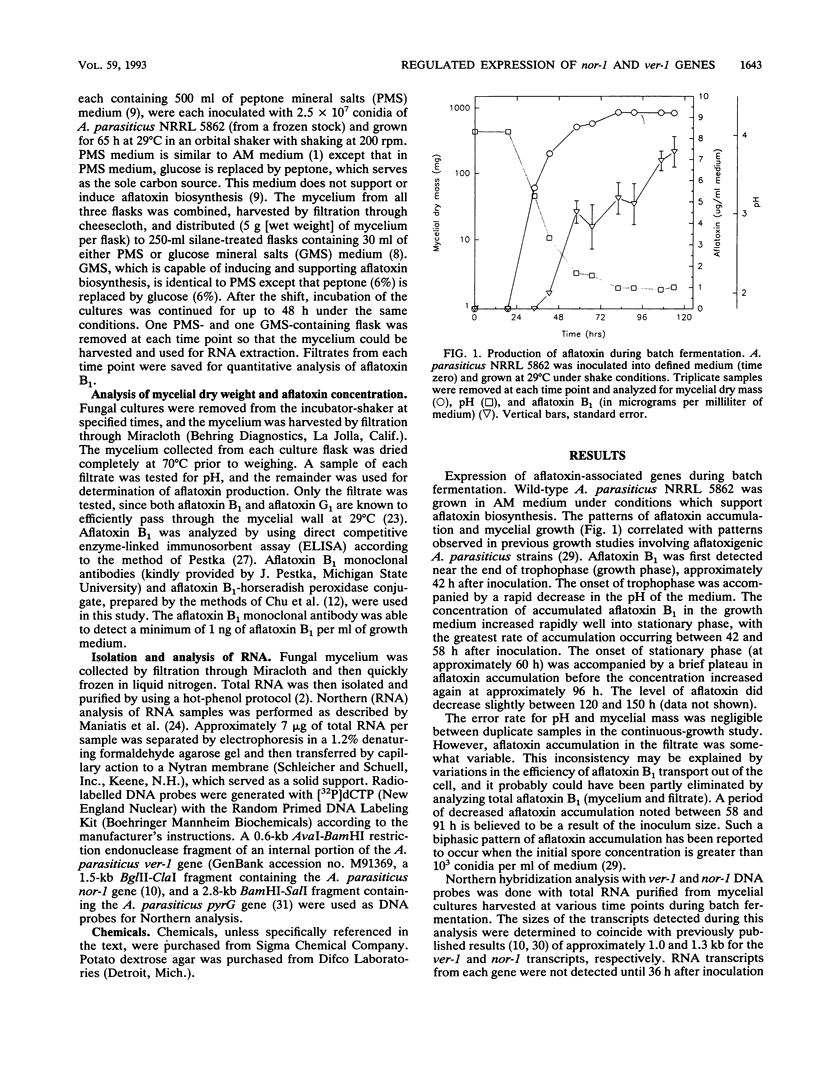
- Yabe K., Nakamura Y., Nakajima H., Ando Y., Hamasaki T. Enzymatic conversion of norsolorinic acid to averufin in aflatoxin biosynthesis. Appl Environ Microbiol. 1991 May;57(5):1340–1345. doi: 10.1128/aem.57.5.1340-1345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]