Abstract
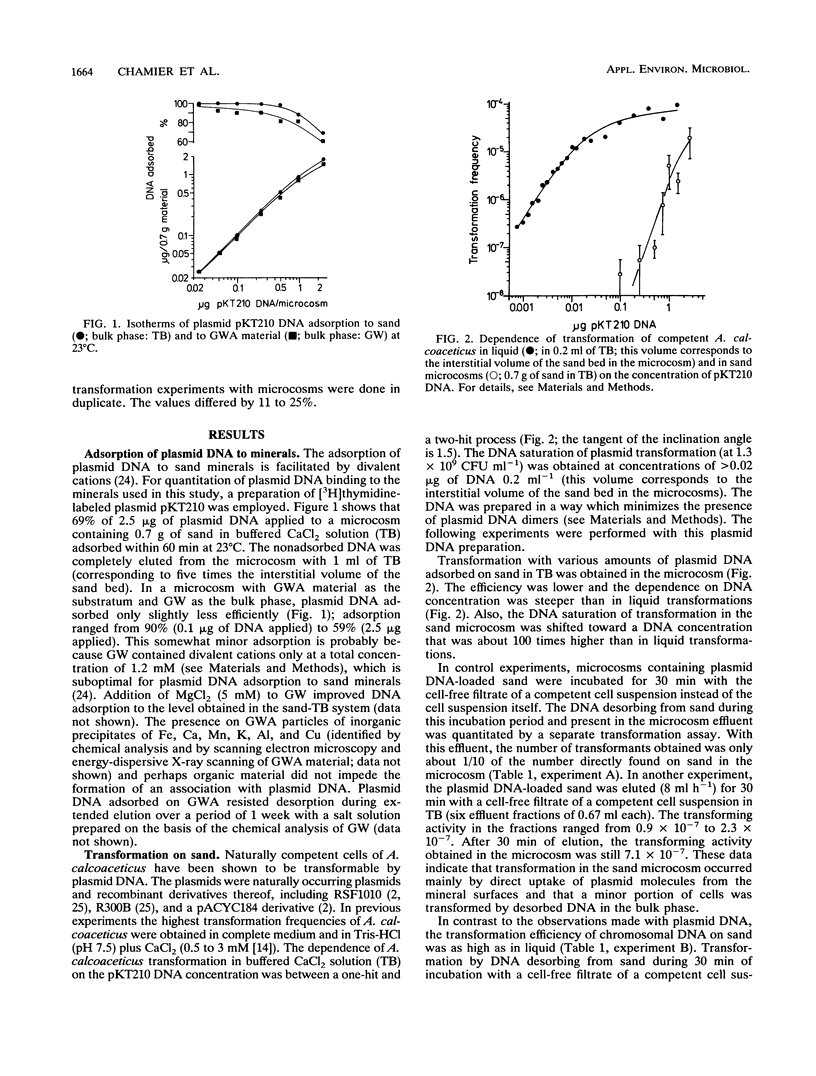
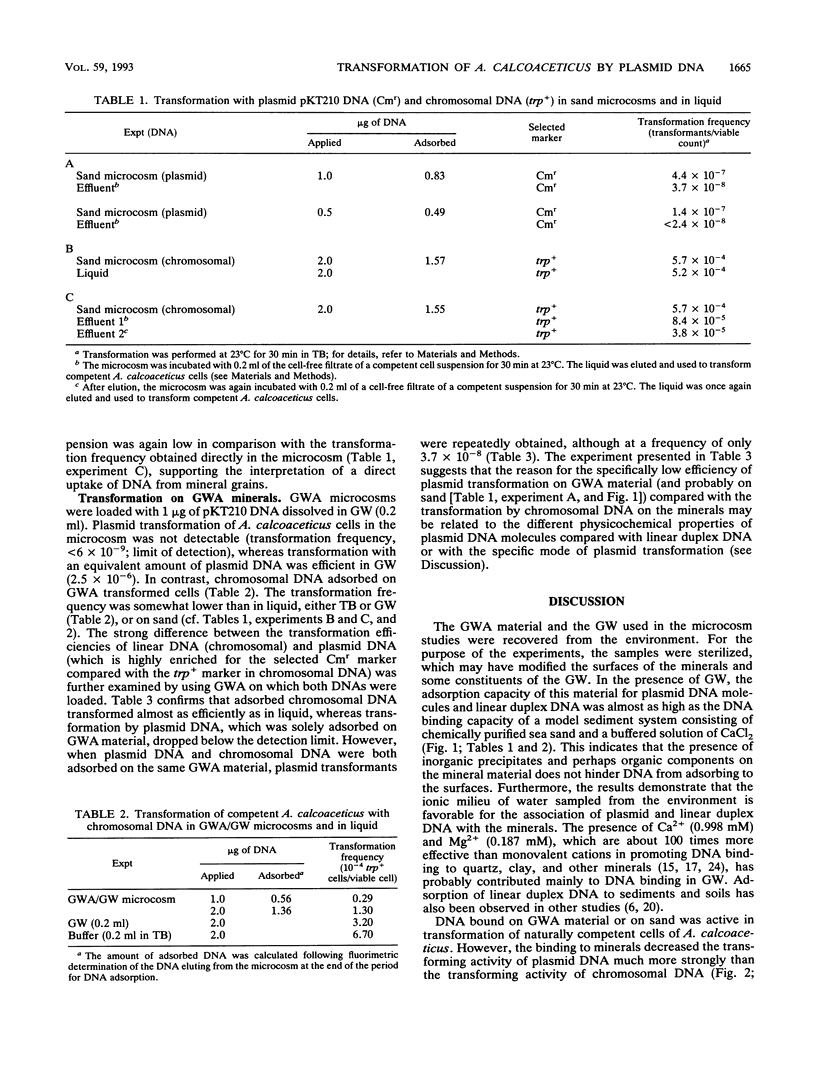
It is known that plasmid DNA and linear duplex DNA molecules adsorb to chemically purified mineral grains of sand and to particles of several clay fractions. It seemed desirable to examine whether plasmid DNA would also adsorb to nonpurified mineral materials taken from the environment and, particularly, whether adsorbed plasmid DNA would be available for natural transformation of bacteria. Therefore, microcosms consisting of chemically pure sea sand plus buffered CaCl2 solution were compared with microcosms consisting of material sampled directly from a groundwater aquifer (GWA) plus groundwater (GW) with respect to the natural transformation of Acinetobacter calcoaceticus by mineral-associated DNA. The GWA minerals were mostly sand with inorganic precipitates and organic material plus minor quantities of silt and clay (illite and kaolinite). The amount of plasmid DNA which adsorbed to GWA (in GW) was about 80% of the amount which adsorbed to purified sand (in buffered CaCl2 solution). Plasmid DNA adsorbed on sand transformed A. calcoaceticus significantly less efficiently than did plasmid DNA in solution. In contrast, the transformation by sand-adsorbed chromosomal DNA was as high as that by DNA in solution. In GWA/GW microcosms, the efficiency of transformation by chromosomal DNA was similar to that in sand microcosms, whereas plasmid transformation was not detectable. However, plasmid transformants were found at a low frequency when GWA was loaded with both chromosomal and plasmid DNA. Reasons for the low transformation efficiency of plasmid DNA adsorbed to mineral surfaces are discussed. Control experiments showed that the amounts of plasmid and chromosomal DNA desorbing from sand during incubation with a cell-free filtrate of a competent cell suspension did not greatly contribute to transformation in sand microcosms, suggesting that transformation occurred by direct uptake of DNA from the mineral surfaces. Taken together, the observations suggest that plasmid DNA and chromosomal DNA fragments which are adsorbed on mineral surfaces in a sedimentary or soil habitat may be available (although with different efficiencies for the two DNA species) for transformation of a naturally competent gram-negative soil bacterium.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna M., Stotzky G. Transformation of Bacillus subtilis by DNA bound on montmorillonite and effect of DNase on the transforming ability of bound DNA. Appl Environ Microbiol. 1992 Jun;58(6):1930–1939. doi: 10.1128/aem.58.6.1930-1939.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Lorenz M. G., Aardema B. W., Wackernagel W. Highly efficient genetic transformation of Bacillus subtilis attached to sand grains. J Gen Microbiol. 1988 Jan;134(1):107–112. doi: 10.1099/00221287-134-1-107. [DOI] [PubMed] [Google Scholar]
- Lorenz M. G., Reipschläger K., Wackernagel W. Plasmid transformation of naturally competent Acinetobacter calcoaceticus in non-sterile soil extract and groundwater. Arch Microbiol. 1992;157(4):355–360. doi: 10.1007/BF00248681. [DOI] [PubMed] [Google Scholar]
- Lorenz M. G., Wackernagel W. Adsorption of DNA to sand and variable degradation rates of adsorbed DNA. Appl Environ Microbiol. 1987 Dec;53(12):2948–2952. doi: 10.1128/aem.53.12.2948-2952.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz M. G., Wackernagel W. Natural genetic transformation of Pseudomonas stutzeri by sand-adsorbed DNA. Arch Microbiol. 1990;154(4):380–385. doi: 10.1007/BF00276535. [DOI] [PubMed] [Google Scholar]
- Romanowski G., Lorenz M. G., Sayler G., Wackernagel W. Persistence of free plasmid DNA in soil monitored by various methods, including a transformation assay. Appl Environ Microbiol. 1992 Sep;58(9):3012–3019. doi: 10.1128/aem.58.9.3012-3019.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanowski G., Lorenz M. G., Wackernagel W. Adsorption of plasmid DNA to mineral surfaces and protection against DNase I. Appl Environ Microbiol. 1991 Apr;57(4):1057–1061. doi: 10.1128/aem.57.4.1057-1061.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. T., van Tuijl J. J., Finnerty W. R. Transformation and mobilization of cloning vectors in Acinetobacter spp. J Bacteriol. 1986 Jan;165(1):301–303. doi: 10.1128/jb.165.1.301-303.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. J., Sinigalliano C. D. Detection of horizontal gene transfer by natural transformation in native and introduced species of bacteria in marine and synthetic sediments. Appl Environ Microbiol. 1990 Jun;56(6):1818–1824. doi: 10.1128/aem.56.6.1818-1824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



