Abstract
Herpes simplex virus type-1 (HSV-1) primarily infects mucoepithelial tissues of the eye and the orofacial region. Subsequently, the virus is retrogradely transported through the axons of the trigeminal sensory neurons to their nuclei, where the virus establishes a lifelong latent infection. During this latency period, the viral genome is transcriptionally silent except for a single region encoding the latency associated transcript (LAT). To understand how HSV-1 latency might affect the expression of substance P in sensory neurons, we transfected primary cultures of trigeminal neurons obtained from rat embryos, with LAT expressing plasmids. The expression of LAT increased the percentage of substance P-immunoreactive neurons by two thirds. To examine the effect of bone morphogenetic protein-7 (BMP7) on the LAT-induced increase in substance P expression in trigeminal neurons, cultures transfected with LAT were treated with BMP7. Treatment with BMP7 reversed the effects of LAT on substance P expression in trigeminal neurons. Our data show for the first time that LAT increases substance P expression in trigeminal neurons and BMP7 can reverse these effects of LAT.
Herpes simplex virus type-1 (HSV-1) is a member of the alphaherpesvirus subfamily. After primary infection of the orofacial region, HSV-1 is retrogradely transported to the nuclei of the trigeminal sensory neurons through their axons that innervate the infected area (19). HSV-1 establishes and maintains a life-long latency in the nuclei of the sensory neurons of the trigeminal ganglia. During latent neural infection with HSV-1, significant gene expression of the viral genome is limited to an area encoding the latency-associated transcript (LAT) (4). LAT is an intron which is processed rapidly from a primary transcript of 8.3 kb to yield shorter transcripts, the most prominent of which is a stable 2kb intron (5).
The role of LAT in latency and reactivation of HSV-1 have been extensively studied (18), and its antiapoptotic effect has been reported from several laboratories (15, 17). Previous studies have shown that latent HSV-1 infection of trigeminal ganglia can alter the expression of many neuronal genes including those involved in the immune response, axonal remodeling, signal transduction and gene expression (11, 10). However, there is little evidence that HSV-1 LAT can affect the expression of neuropeptides in trigeminal sensory neurons.
Substance P is an 11 amino acid residue neuropeptide which was the first inflammatory neuropeptide discovered. It is detected in both the central and the peripheral nervous systems, especially in peripheral sensory neurons (1). Substance P is a member of the tachykinin family and has a role in neurogenic inflammation, modulation of immune response and pain sensation (14). Previous studies have shown that substance P levels are increased in various inflammatory and infectious diseases (13).
TGF-β family members, including bone morphogenetic protein-7 (BMP7), are among the factors affecting neuropeptide expression in sensory neurons (8). There is recent evidence that LAT can antagonize the effects of TGF-β family members (7). In addition, we recently found that LAT inhibits BMP7-induced dendritic growth in sympathetic neurons (9). These data suggest that an interaction may exist between BMP7 and LAT. In this study, we examined the effect of HSV-1 LAT on substance P expression via immunoreactivity in cultured trigeminal neurons from rat embryos. We also asked if BMP7 can alter the effects of LAT on substance P expression.
Human recombinant BMP7 was generously provided by Curis (Cambridge, MA). Cytosine- β-D-arabinofuranoside (ara-c) was purchased from Sigma Chemical Corp. (St Louis, MO). A plasmid coding enhanced green fluorescence protein (pEGFP-C1) was purchased from Clontech (Clontech, Palo Alto, CA). A plasmid expressing the full length 2.0 kb LAT intron as a lariat was a generous gift from Dr. Lawrence Feldman (UCLA, LA, CA).
Trigeminal ganglia and dorsal root ganglia were dissected from embryos (E19 and E14, respectively) of Holtzman rats (Harlan Sprague Dawley, Rockford, IL). The cells were dissociated after treatment with trypsin (2.5 mg/ml) and collagenase (1mg/ml) for 35 minutes. Cells were plated on poly-D-lysine-coated (200 μg/ml) coverslips and maintained on serum-free medium containing β-nerve growth factor (NGF: 100 ng/ml). One day later, the cultures were treated with medium containing 1μM of the antimitotic drug cytosine- β-D-arabinofuranoside (ara-c) (Sigma Chemical Corp, St Louis, MO) for 2 days to eliminate non-neuronal cells, and then the culture were allowed to recover for two days before transfection.
Cells were cotransfected with a plasmid encoding the enhanced green fluorescence protein (pEGFP-C1, Clontech, Palo Alto, CA) and the expression vector LTF900 containing the LAT 2.0 kb insert. In control experiments, cells were cotransfected with pEGFP-C1 and the pCDNA3.1 vector (Invitrogen, CA). Transfections were performed using Lipofectamine 2000 (Invitrogen, CA). Briefly, cells were treated with 150 μl of DMEM containing 1.6 μg of plasmid DNA and 6 μg of lipofectamine for six hours. Subsequently, cells were washed and allowed to recover for 2 days.
Antisense and sense riboprobes for LAT were prepared as follows: a fragment of LAT (814 basepairs) was amplified by PCR, and then subcloned into the PCR II-TOPO vector (invitrogen, CA). Digoxigenin-labeled riboprobes were generated by in vitro transcription according to the manufacturer’s instructions (Roche, Indianapolis, ID). Cultures were fixed with 4% formaldehyde for 1 hour and washed twice with 2X SSC. Cultures were treated with proteinase K (5 μg/ml) for 10 minutes then washed twice with 2X SSC. Cells were incubated at 50° C for 3 hours with a pre-hybridization solution which contained 50 % formamide, 4X SSC, 1 mM EDTA, 1X Denhardt’s solution, 10% dextran sulfate, yeast tRNA (250 μg/ml) and denatured salmon sperm DNA (250 μg/ml). Cells were hybridized by incubation at 50° C for 16 hours with 2μg/ml of probe in hybridization solution. The LAT signal was detected using anti-digoxigenin Fab fragment conjugated to rhodamine (Roche, Indianapolis, ID) and visualized by fluorescence microscopy.
Expression of substance P in cultured neurons was detected by immunostaining. Cells were fixed with 4% paraformaldehyde for 20 minutes, and permeabilized with 0.1% Triton X-100 for 4 minutes. This was followed by incubation with 5% bovine serum albumin for 2 hours at room temperature. Cells were reacted with rabbit polyclonal antibodies to substance P (ImmunoStar Inc., Hudson, WI) overnight, and then with anti-rabbit rhodamine conjugated secondary antibodies (Boehringer Mannheim, West Grove, PA), and visualized by fluorescence microscopy.
For the assessment of neuronal survival, dead neuronal cells were detected through the recognition of nuclear morphology by incubating the cells with Hoechst stain (Bisbenzimide, Sigma, St. Louis, MO) for 20 minutes.
Data in the text are presented as mean ± SEM. Statistical significance was determined by a one-way ANOVA test followed by Tukey’s post hoc test, or a two-tailed unpaired t-test.
LAT was detected in the nuclei of 88.6 % of cultured trigeminal neurons co-transfected with plasmids containing EGFP and LAT (31 out of total of 35 neurons) (9). To study the effect of LAT on substance P expression in cultured trigeminal neurons co-transfected with plasmids containing EGFP and LAT or EGFP and the pCDNA3.1 vector, the neurons were immunostained for substance P (Fig. 1C–H). We found that substance P is expressed by trigeminal neurons of different sizes. A significant increase in the percentage of substance P- immunoreactive neurons was detected in the LAT-transfected neurons (31.8%) when compared with the pCDNA3.1-transfected neurons (18.2%) (Fig. 1I). We next examined if the LAT-induced increase of substance P immunoreactivity in trigeminal neurons extends to DRG neurons. We found that LAT did not increase the percentage of substance P-immunoreactive DRG neurons (17.4± 8 % in LAT transfected neurons in comparison with 17.1± 3.1 in control neurons), which indicates that the effect of LAT is specific to trigeminal neurons.
Figure 1. Expression of HSV-1 LAT increases the percentage of substance P-immunoreactive trigeminal neurons.
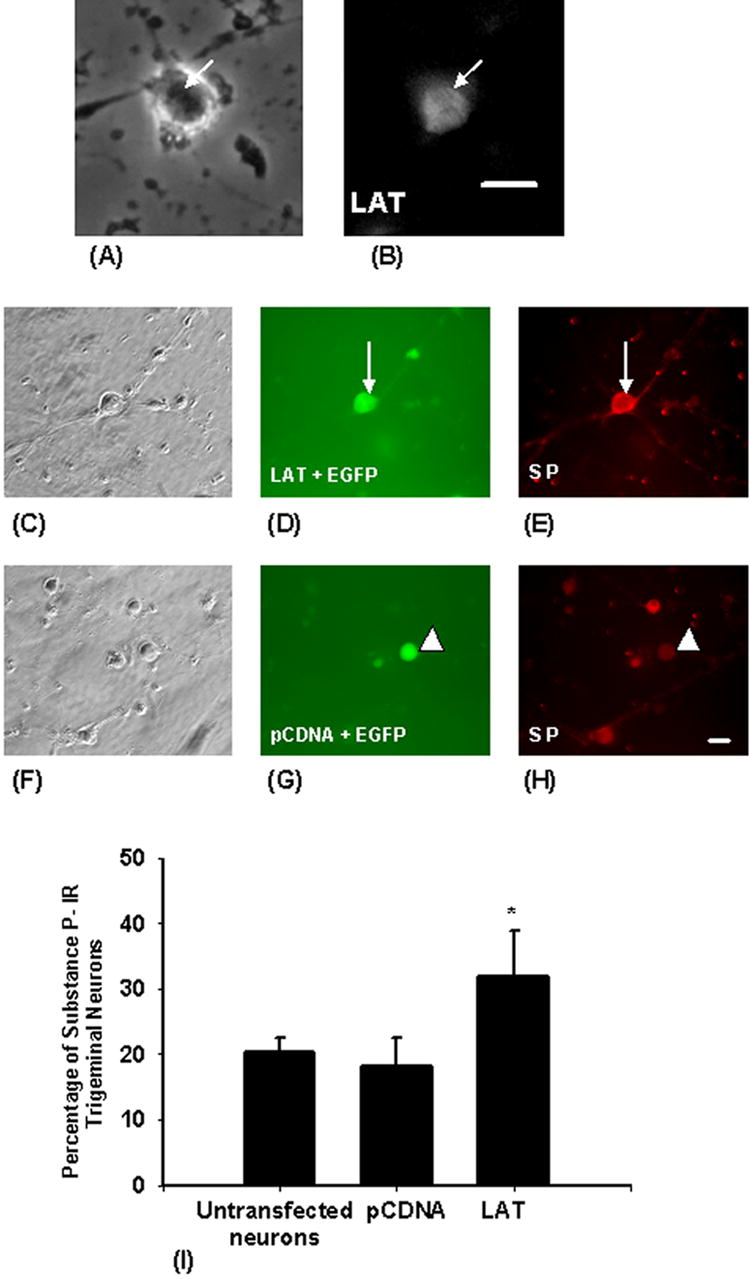
In order to Express LAT in trigeminal neurons, cultured trigeminal neurons were cotransfected with plasmids expressing LAT and EGFP as a marker. The upper panel shows a trigeminal neuron transfected with LAT containing plasmid (A), and LAT was detected in the nucleus of the neuron (indicated by arrows) by in situ hybridization (B). Two days after transfection, the cells were fixed and immunostained for substance P. The middle panel shows a trigeminal neuron (indicated by arrows) cotransfected with LAT and EGFP (D) and this neuron was substance P-immunoreactive (E). The lower panel shows a trigeminal neuron (indicated by arrowheads) cotransfected with pCDNA3.1 and EGFP (G) and that neuron showed only background staining (not immunoreactive to substance P) (H). LAT expression resulted in a two-thirds increase in the percentage of substance P immunoreactive trigeminal neurons (I). Bars are means ± S.E.M. (∗) indicates p < 0.05. Scale bar = 40μM.
Recent findings provided evidence that LAT encodes a microRNA that interferes with the signaling pathway of TGF-β (6). Based on this, we studied the effect of BMP7 on the LAT- induced substance P immunoreactivity in trigeminal neurons. Treatment with BMP7 (30 ng/ml) for 6 days reversed the effect of LAT significantly decreasing the percentage of substance P-immunoreactive neurons from 31.8% to 11.4% (Fig. 2A) and the effect of BMP7 was dose-dependent (Fig. 2B). In order to exclude the possibility that BMP7 may decrease substance P immunoreactivity by decreasing the viability of trigeminal neurons, cultured trigeminal neurons were stained with Hoechst dye to detect dead neurons. No significant difference in neuronal survival between cultures treated with medium or cultures treated with medium supplemented with BMP7 (30 ng/ml) was observed (Fig. 2C).
Figure 2. BMP7 reverses the LAT-induced increase in the percentage of substance P-immunoreactive trigeminal neurons.
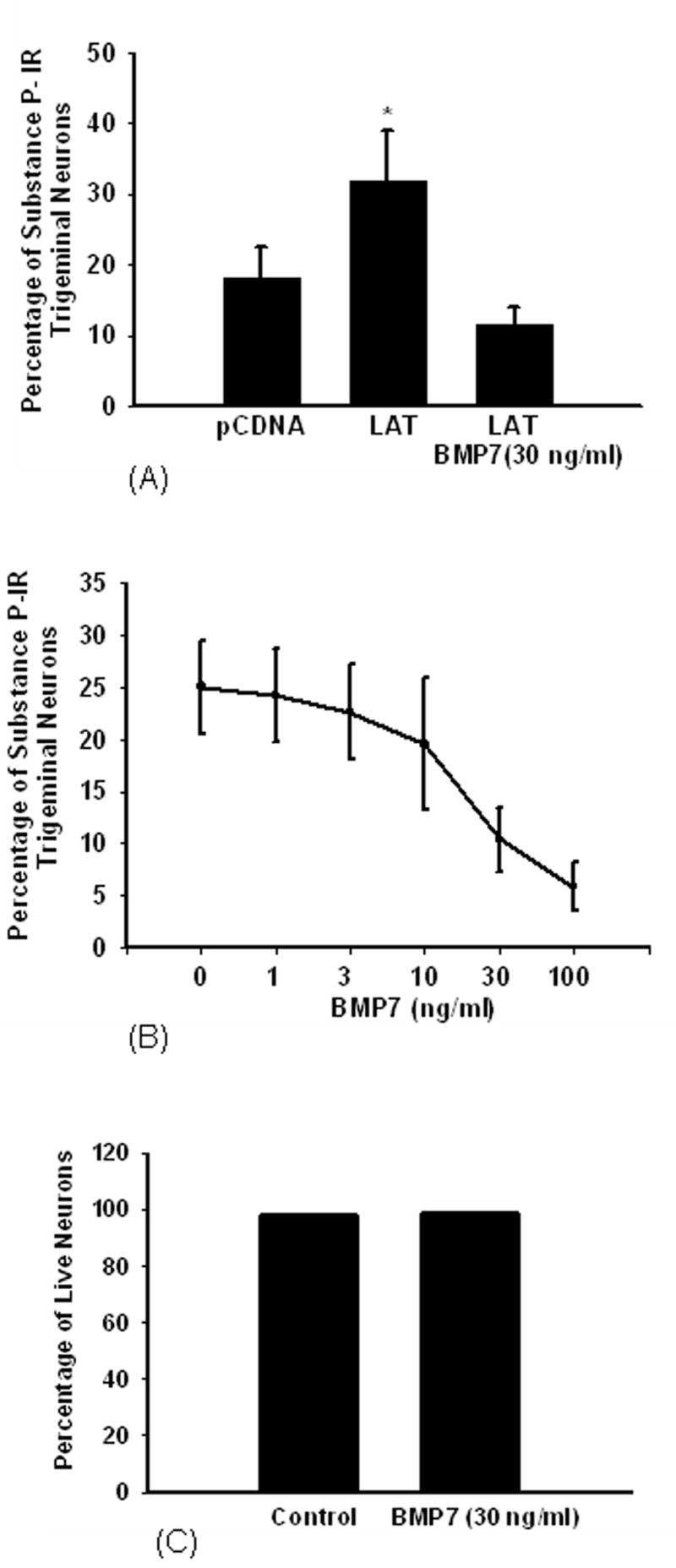
Cultured trigeminal neurons were cotransfected with plasmids expressing EGFP and LAT or the empty vector pCDNA3.1. Two days later, cells transfected with LAT were treated with medium containing BMP7(30 ng/ml) for 6 days or with medium only. Cells were fixed and immunostained for substance P. BMP7 reversed the LAT-induced increase in the percentage of substance P-immnuoreactive trigeminal neurons (A). The effect of BMP7 on LAT-induced increase in substance P immunoreactivity was dose dependent and reduced the percentage of substance P-immunoreactive trigeminal neurons by more than 2-fold as compared to LAT expressing neurons (B) (*p< 0.01). BMP7 treatment did not affect the survival of trigeminal neurons (C).
This data presented indicates that the expression of HSV-1 LAT in trigeminal neurons increased substance P expression. This finding is novel and interesting. There is currently no information in the literature indicating that latent HSV-1 infection of neurons or the expression of HSV-1 LAT can upregulate the expression of substance P. Previous studies have shown that induction of latent HSV-1 infection in the trigeminal ganglia of mice through ocular inoculation with HSV-1 can alter the expression of many neuronal genes, including those genes involved in the immune response, axonal remodeling, signal transduction and gene expression (11, 10). Our findings indicate that expression of LAT in trigeminal neurons can upregulate the expression of substance P. In contrast to the effect on trigeminal neurons, LAT did not increase substance P immunoreactivity in DRG neurons, which indicates that the effect of LAT on substance P expression is specific for trigeminal neurons. The specificity of LAT action on trigeminal neurons may help in understanding the reason that the primary location for latent HSV1 infection is the trigeminal neurons. Our findings are in agreement with the previous report that LAT was found to confer site-specificity of reactivation, and substitution of HSV-1 LAT with HSV-2 LAT sequences resulted in reduction of activation from infected rabbit trigeminal neurons (20).
Substance P is an important mediator of neurogenic inflammation and neuroimmunomodulation (2, 13). It has been found to induce edema by increasing microvascular permeability, as well as mediating pain sensation in peripheral sensory neurons (1). The HSV-1 LAT-induced increase of substance P expression in trigeminal neurons may explain the sensory changes associated with HSV-1 infection. Previous studies have shown that mice latently infected with HSV-1 were found to have post herpetic pain (16). Allodynia was also observed following inoculation of HSV-1 in the footpad in rats (3). In addition, there is evidence from clinical studies that post herpetic neuralgia has been reported in patients with recurrent HSV-1 infection after the disappearance of facial manifestations (12, 6). Substance P upregulation during the latency of HSV-1 in trigeminal neurons is a possible mechanism for these neuralgias. We recently found that LAT and HSV-1 latency inhibit bone morphogenetic protein-7 (BMP7)–induced dendritic growth in cultured sympathetic neurons (9). The mechanism(s) through which LAT produces its effects is not completely understood. There are recent findings that LAT encodes a microRNA, and this microRNA interferes with the TGF-β signaling pathway (7). These findings suggest that LAT may regulate the expression of neuronal genes through a microRNA mechanism. Although the LAT-containing plasmid used in our study did not contain the sequence encoding the microRNA, we observed an effect of LAT on substance P expression. This finding is supported by the observation that deletion of the sequence encoding the microRNA did not completely abolish the effects of LAT (7). These findings indicate that other sequences in LAT downstream to the sequence encoding the microRNA also have effects on sensory neurons.
The ability of BMP7 to reverse the HSV-1 LAT-induced increase in substance P immunoreactivity in trigeminal neurons is also novel and interesting. Previous work showed that members of the TGF-β family can regulate the expression of neuropeptides in sensory neurons (8). On the other hand, recent studies showed evidence that there is an interaction between HSV-1 LAT and TGF-β family members, which includes BMP7 (7). BMP7 produces its effects through a signaling pathway that is very similar to TGF-β, and this suggests that LAT also interferes with BMP7 signaling. This suggestion was further supported by the findings from our laboratory that LAT inhibited BMP7-induced dendritic growth in sympathetic neurons (9).
Our results indicate that substance P may have a role in latency of HSV-1 in trigeminal neurons, and this role may include the establishment and maintenance of latency (via immunomodulation), or the pathogenesis of the manifestations associated with latent HSV-1 infection of trigeminal neurons. Targeting substance P synthesis or its signaling pathway may interfere with HSV-1 latency or improve associated symptoms. The BMP7 reversal of HSV-1 LAT-induced increase in substance P expression in trigeminal neurons opens the possibility of a role for BMP7 (and probably other TGF-β family members) in the management of reactivation of latent HSV-1 infection by mitigating post-herpetic pain.
Acknowledgments
This article is dedicated to the memory of Dr. Dennis Higgins.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brain SD, Cox HM. Neuropeptides and their receptors: innovative science providing novel therapeutic targets. Br J Pharmacol. 2006;147(Suppl 1):S202–11. doi: 10.1038/sj.bjp.0706461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–4. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 3.Dalziel RG, Bingham S, Sutton D, Grant D, Champion JM, Dennos SA, Quinn JP, Bountra C, Mark MA. Allodynia in rats infected with varicella zoster virus- a small animal model for post-herpetic neuralgia. Brain Res Rev. 2004;46:234–42. doi: 10.1016/j.brainresrev.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Deatly AM, Spivack JG, Lavi E, Fraser NW. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci U S A. 1987;84:3204–8. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farrell MJ, Dobson AT, Feldman LT. Herpes simplex virus latency-associated transcript is a stable intron. Proc Natl Acad Sci U S A. 1991;88:790–4. doi: 10.1073/pnas.88.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzales GR. Postherpes simplex type 1 neuralgia simulating postherpetic neuralgia. J Pain Symptom Manage. 1992;7:320–3. doi: 10.1016/0885-3924(92)90065-p. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, Gartner JJ, Sethupathy P, Hatzigeorgiou AG, Fraser NW. Anti-apoptotic function of a microRNA encoded by the HSV-1 latency-associated transcript. Nature. 2006;442:82–5. doi: 10.1038/nature04836. [DOI] [PubMed] [Google Scholar]
- 8.Hall AK, Dinsio KJ, Cappuzzello J. Skin cell induction of calcitonin gene-related peptide in embryonic sensory neurons in vitro involves activin. Dev Biol. 2001;229:263–70. doi: 10.1006/dbio.2000.9966. [DOI] [PubMed] [Google Scholar]
- 9.Hamza MA, Higgins DM, Ruyechan WT. Herpes simplex virus type-1 latency inhibits dendritic growth in sympathetic neurons. Neurobiol Dis. doi: 10.1016/j.nbd.2006.07.011. (In press) [DOI] [PubMed] [Google Scholar]
- 10.Kent JR, Fraser NW. The cellular response to herpes simplex virus type 1 (HSV-1) during latency and reactivation. J Neurovirol. 2005;11:376–83. doi: 10.1080/13550280591002405. [DOI] [PubMed] [Google Scholar]
- 11.Kramer MF, Cook WJ, Roth FP, Zhu J, Holman H, Knipe DM, Coen DM. Latent herpes simplex virus infection of sensory neurons alters neuronal gene expression. J Virol. 2003;77:9533–41. doi: 10.1128/JVI.77.17.9533-9541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krohel GB, Richardson JR, Farrell DF. Herpes simplex neuropathy. Neurology. 1976;26:596–7. doi: 10.1212/wnl.26.6.596. [DOI] [PubMed] [Google Scholar]
- 13.Lecci A, Maggi CA. Peripheral tachykinin receptors as potential therapeutic targets in visceral diseases. Expert Opin Ther Targets. 2003;7:343–62. doi: 10.1517/14728222.7.3.343. [DOI] [PubMed] [Google Scholar]
- 14.Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, Maggi CA. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74:1445–63. doi: 10.1016/j.lfs.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 15.Perng GC, Jones C, Ciacci-Zanella J, Stone M, Henderson G, Yukht A, Slanina SM, Hofman FM, Ghiasi H, Nesburn AB, Wechsler SL. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science. 2000;287:1500–3. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- 16.Takasaki I, Sasaki A, Andoh T, Nojima H, Shiraki K, Kuraishi Y. Effects of analgesics on delayed postherpetic pain in mice. Anesthesiology. 2002;96:1168–74. doi: 10.1097/00000542-200205000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Thompson RL, Sawtell NM. Herpes simplex virus type 1 latency-associated transcript gene promotes neuronal survival. J Virol. 2001;75:6660–75. doi: 10.1128/JVI.75.14.6660-6675.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson RL, Sawtell NM. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J Virol. 1997;71:5432–40. doi: 10.1128/jvi.71.7.5432-5440.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren KG, Brown SM, Wroblewska Z, Gilden D, Koprowski H, Subak-Sharpe J. Isolation of latent herpes simplex virus from the superior cervical and vagus ganglions of human beings. N Engl J Med. 1978;298:1068–9. doi: 10.1056/NEJM197805112981907. [DOI] [PubMed] [Google Scholar]
- 20.Yoshikawa T, Hill JM, Stanberry LR, Bourne N, Kurawadwala JF, Krause PR. The characteristic site-specific reactivation phenotypes of HSV-1 and HSV-2 depend upon the latency-associated transcript region. J Exp Med. 1996;184:659–64. doi: 10.1084/jem.184.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]


