Abstract
Objective
To test whether beta carotene affects age-related maculopathy (ARM) in a large-scale randomized trial.
Design
Randomized, double-masked, placebo-controlled trial among 22,071 apparently healthy male U.S physicians aged 40 to 84 years. Participants were randomly assigned to receive beta carotene (50 mg on alternate days) or placebo.
Main Outcome Measure
Incident ARM responsible for a reduction in best-corrected visual acuity to 20/30 or worse.
Results
After 12 years of treatment and follow-up, there were 162 cases of ARM in the beta carotene group and 170 cases in the placebo group (relative risk [RR], 0.96; 95 percent confidence interval [CI], 0.78 to 1.20). The results were similar for the secondary endpoints of ARM with or without vision loss (275 cases versus 274 cases; RR, 1.01; CI, 0.86 to 1.20) and advanced ARM (63 cases versus 66 cases; RR, 0.97; CI, 0.69 to 1.37).
Conclusions
These randomized data with 12 years of treatment in a large population of apparently healthy men indicate that beta carotene supplementation has no beneficial or harmful effect on incidence of ARM.
Applications to Clinical Practice
Long-term supplemental use of beta carotene neither decreases nor increases the risk of ARM.
Introduction
In the United States (U.S), an estimated 30% of persons aged 65 years and over show signs of early age-related maculopathy (ARM), or its late-stage manifestations also known as age-related macular degeneration (AMD) (1, 2). Despite its common occurrence, however, there are no generally effective treatment options. Laser photocoagulation (3, 4), photodynamic therapy (5, 6), and anti-vascular endothelial growth factor therapy (7, 8) are available, but only for a minority of persons with the late-stage, neovascular form of the disease. A majority of the affected individuals have the earlier, atrophic form of ARM which has no known proven treatment. For these reasons, identification of preventive measures is of particular clinical and public health importance.
In some, but not all, observational studies, individuals who self-select for antioxidant nutrients and other dietary factors have lower risks of ARM (9–12). Evidence from randomized trials is accumulating to evaluate a possible role for nutritional supplements in delaying disease onset and progression. The Age-related Eye Disease Study (AREDS) demonstrated that treatment with high-dose antioxidant supplements (vitamin C, vitamin E, and beta carotene) and zinc could reduce the risk of progression to advanced ARM in persons at high risk (13). These findings in AREDS are an important advance in disease prevention and serve as the basis for current recommendations regarding nutritional supplements in persons at high risk for ARM (14). Nonetheless, many important questions remain. For example, some ophthalmologists, citing possible adverse effects of some components in the AREDS formulation, have suggested the use of alternative formulations that, nonetheless, remain largely unproven (15). In addition, the value of antioxidant supplements in persons at usual risk of ARM could not be evaluated in AREDS and remains to be determined. These and other unresolved issues of clinical importance can be addressed with further information on the specific effects of each component of the AREDS formulation, as well as the effects of other nutrient combinations, in persons at usual risk of ARM, and in those at increased risk of advanced ARM.
Beta carotene was included in the AREDS formulation because at the start of that trial it was readily available in a research formulation and was already under investigation in several large randomized trials of cancer and cardiovascular disease. One of these was the Physicians’ Health Study I (PHS I) which tested beta carotene and low-dose aspirin among 22,071 apparently healthy U.S. male physicians. The main trial results for PHS I showed no benefit or harm of 12 years of beta carotene treatment on cancer or cardiovascular disease endpoints (16). In this report we describe the results for ARM during the randomized beta carotene component of PHS I.
Methods
Study Population
The Physicians’ Health Study I (PHS I) was a randomized, double-masked, placebo-controlled trial of beta-carotene (Lurotin, BASF Corporation) (50-mg supplement every other day) and low-dose aspirin (Bufferin, Bristol-Myers) (325 mg every other day) in the primary prevention of cardiovascular disease and cancer, among 22,071 apparently healthy U.S. male physicians aged 40–84 years in 1982. Information collected at baseline included height, weight, history of cigarette smoking, alcohol use, blood pressure, cholesterol, history of diabetes mellitus, physical activity, and multivitamin use. Participants completed annual follow-up questionnaires supplying information about their compliance with the treatment regimens and the occurrence of any relevant events. Informed consent was obtained from all participants, and the research protocol was reviewed and approved by the Institutional Review Board at Brigham and Women’s Hospital in Boston.
The randomized aspirin component of PHS I was terminated early on January 25, 1988, due primarily to a statistically significant (P<0.001) 44 percent reduction in the risk of a first myocardial infarction in the aspirin group (17). The randomized beta carotene component continued uninterrupted until its scheduled termination, on December 31, 1995, with an average duration of treatment of 12 years (range, 11.6 to 14.2) (16). At the end of 11 years of follow-up (the last year completed for all participants), 99.2 percent were still providing information on morbidity, and follow-up for mortality was 99.9 percent complete. Eighty percent of participants in both the beta carotene group and the placebo group were still taking the study pills, with an average compliance among pill takers of over 97 percent. Thus, even after 11 years, 78 percent of the study pills in the beta carotene group were reported as still being taken. In the placebo group, 6 percent reported taking supplemental beta carotene or vitamin A. The validity of reported compliance with the assigned treatment was assessed by measuring plasma beta carotene concentrations in blood obtained at unannounced visits to a small sample of participants in three geographic areas. Those assigned to receive beta carotene had significantly higher mean concentrations than those given placebo (1.2 vs. 0.3 mg per liter [2.24 vs. 0.56 mmol per liter], P<0.001) (18).
Ascertainment and Definition of ARM Endpoints
Information on diagnoses of ARM made during the first seven years of the trial was requested on the 84-month questionnaire. Physicians were asked "Have you ever had macular degeneration diagnosed in your right (left) eye?" If yes, they were requested to provide the month and year of the diagnosis. Subsequent annual questionnaires requested information on diagnoses during the preceding year. Signed permission to examine medical records pertaining to the diagnosis was requested on the questionnaires and, when necessary, in separate follow-up mailings. Ophthalmologists and optometrists were contacted by mail and requested to complete an ARM questionnaire supplying information about the date of initial diagnosis, the best-corrected visual acuity at the time of diagnosis, and the date when best-corrected visual acuity reached 20/30 or worse (if different from the date of initial diagnosis). Information was also requested about signs of ARM observed (drusen, retinal pigment epithelium [RPE] hypo/hyperpigmentation, geographic atrophy, RPE detachment, subretinal neovascular membrane, or disciform scar) when visual acuity was first noted to be 20/30 or worse, and the date when exudative neovascular disease, if present, was first noted (defined by presence of RPE detachment, subretinal neovascular membrane, or disciform scar). We also asked whether there were other ocular abnormalities that would explain or contribute to visual loss and if so, whether the ARM, by itself, was significant enough to cause the best-corrected visual acuity to be reduced to 20/30 or worse. Medical records were obtained for more than 92% of participants reporting ARM.
The primary endpoint was visually-significant ARM defined as a self-report confirmed by medical record evidence of an initial diagnosis subsequent to randomization but before December 31, 1995, with best-corrected vision loss to 20/30 or worse attributable to ARM. Two secondary endpoints were also defined: ARM with or without vision loss, comprised of all incident cases, and advanced ARM, comprised of those cases of visually-significant ARM with pathological findings of geographic atrophy, RPE detachment, subretinal neovascular membrane, or disciform scar. The latter endpoint was defined in order to provide a more direct comparison of our results with the findings for advanced AMD in AREDS.
The present report includes the 21,142 participants who were followed for at least seven years and provided information on diagnoses of ARM made during the first seven years of the trial on the 84-month questionnaire (i.e. physicians who died during the first seven years of follow-up, and therefore did not respond to the 84-month questionnaire, were excluded). Of these, 10,585 were in the beta carotene group and 10,557 were in the placebo group.
Data Analysis
The Cox proportional-hazards model was used to estimate the relative risk of ARM among those assigned to beta carotene compared with those assigned placebo after adjustment for age (years) at baseline and randomized aspirin treatment. Models were also fit separately within four age groups; 40–49, 50–59, 60–69, 70–84 years. Tests of trend of the effect of age on any association between beta carotene and ARM were calculated by including a term for the interaction of beta carotene and age (expressed as a continuous variable with values 1 to 4 corresponding to the four age groups) in a proportional hazards model. For each relative risk, the 95 percent confidence interval and two-sided P value were calculated. All analyses were conducted with SAS version 8.2 (SAS Institute, Cary, NC).
We also analyzed subgroup data according to baseline categories of smoking (current, not current), alcohol use (daily, weekly, rarely), body mass index (kg/m2) (<25.0, 25.0–29.9, ≥30.0), hypertension (defined as reported systolic blood pressure of 140 mm Hg or higher, diastolic blood pressure of 90 mm Hg or higher, or history of treatment for high blood pressure) (yes, no), high cholesterol (defined as ≥240 mg/dL or history of past or current lipid-lowering treatment) (yes, no), and multivitamin use (yes, no) to explore possible modification of any effect of beta carotene. Tests of interaction were performed to evaluate the statistical significance of any modifying effect of these variables. We also used an interaction term with length of follow-up to test for a trend of the relative risk over time and to evaluate the adequacy of the proportional hazards assumption over time.
Individuals, rather than eyes, were the unit of analysis because eyes were not examined independently, and participants were classified according to the status of the worse eye as defined by disease severity. When the worse eye was excluded because of visual acuity loss attributed to other ocular abnormalities the fellow eye was considered for classification.
Results
Baseline characteristics in the beta carotene and placebo groups are shown in Table 1. As expected in a large randomized trial, these characteristics were equally distributed between the two groups.
Table 1.
Baseline Characteristics in Randomized Beta-Carotene and Placebo Treatment Groups in the Physicians’Health Study.*
| Characteristics | Beta-Carotene (n=10,585) | Placebo (n=10,557) |
|---|---|---|
| Mean age, y | 52.8 | 52.8 |
| 40–49 | 42.3 | 42.2 |
| 50–59 | 34.2 | 34.2 |
| 60–69 | 17.9 | 17.9 |
| 70–84 | 5.5 | 5.7 |
| Medical history | ||
| Reported hypertension† | 22.8 | 23.1 |
| Reported high cholesterol level‡ | 11.6 | 12.3 |
| Reported diabetes mellitus | 2.0 | 2.3 |
| Mean body mass index, mean (SD)§ | 24.8 | 24.8 |
| Parental history of myocardial infarction| | 12.7 | 13.5 |
| Health habits | ||
| Cigarette smoking | ||
| Never | 50.2 | 50.0 |
| Past only | 39.1 | 39.4 |
| Current | 10.7 | 10.6 |
| Alcohol use | ||
| Daily | 24.3 | 24.6 |
| Weekly | 50.1 | 49.1 |
| Rarely | 25.6 | 26.4 |
| Physical activity¶ | 27.4 | 27.5 |
| Multivitamin use (current) | 19.1 | 19.8 |
Data are given as the percentage of participants, unless otherwise indicated.
Hypertension is defined as reported systolic blood pressure of 140 mm Hg or higher, diastolic blood pressure of 90 mm Hg or higher, or history of treatment for high blood pressure.
High cholesterol level is defined as reported high cholesterol, a reported high blood cholesterol level of 240 mg/dL, or history of treatment with cholesterol-lowering medication.
Body mass index is weight in kilograms divided by height in meters squared.
Myocardial infarction in either parent before age 60 years.
Reported vigorous exercise once or more per week.
During an average of 12 years of treatment and follow-up, a total of 549 cases of ARM were confirmed. These included 332 cases of visually-significant ARM, approximately two-thirds of which were characterized by some combination of drusen and RPE changes at the time vision was first noted to be 20/30 or less. One-hundred twenty nine of these visually-significant cases developed signs of advanced ARM during follow-up.
There was no statistically significant benefit or harm of beta carotene supplementation on any ARM endpoint (Table 2). For the primary endpoint of visually-significant ARM, there were 162 cases in the beta carotene group and 170 cases in the placebo group (relative risk [RR], 0.96; 95 percent confidence interval [CI], 0.78 to 1.20). Similar findings were observed for the endpoints of ARM with or without vision loss (275 cases in the beta carotene group versus 274 in the placebo group; RR, 1.01; CI, 0.86 to 1.20) and advanced ARM (63 cases in the beta carotene group versus 66 in the placebo group; RR, 0.97; CI, 0.69 to 1.37). For all 3 ARM endpoints, RRs did not vary significantly over the four age groups (P interaction, each > 0.7).
Table 2.
Confirmed Cases of ARM According to Beta-Carotene Treatment Assignment in Physicians’ Health Study I.
| Endpoint | Beta Carotene (N=10,585) | Placebo (N=10,557) | RR* | (95% CI) |
|---|---|---|---|---|
| Visually significant ARM | 162 | 170 | 0.96 | 0.78–1.20 |
| ARM with or without vision Loss | 275 | 274 | 1.01 | 0.86–1.20 |
| Advanced ARM | 63 | 66 | 0.97 | 0.69–1.37 |
Abbreviations: ARM, age-related maculopathy; RR, relative risk; CI, confidence interval.
Adjusted for aspirin treatment assignment
The figure presents the cumulative incidence rates of visually significant ARM, ARM with or without visual acuity loss, and advanced ARM according to the year of follow-up. There were no significant effects of beta carotene on any of the ARM endpoints after excluding cases diagnosed during the first 2 years (visually-significant ARM: RR, 0.93; CI, 0.74 to 1.16; ARM with or without vision loss: RR, 0.99; CI, 0.83 to 1.17; advanced ARM: RR, 0.95; CI, 0.66 to 1.37) or first 5 years (visually-significant ARM: RR, 0.93; CI, 0.73 to 1.19; ARM with or without vision loss: RR, 1.06; CI, 0.88 to 1.27; advanced ARM: RR, 0.84; CI, 0.55 to 1.28) of follow-up. Tests of proportionality of the hazard ratio over time indicated no statistically significant trend over time for any ARM endpoint (visually-significant ARM, p=0.16; ARM with or without vision loss, p=0.99; advanced ARM, p=0.19).
Figure 1.
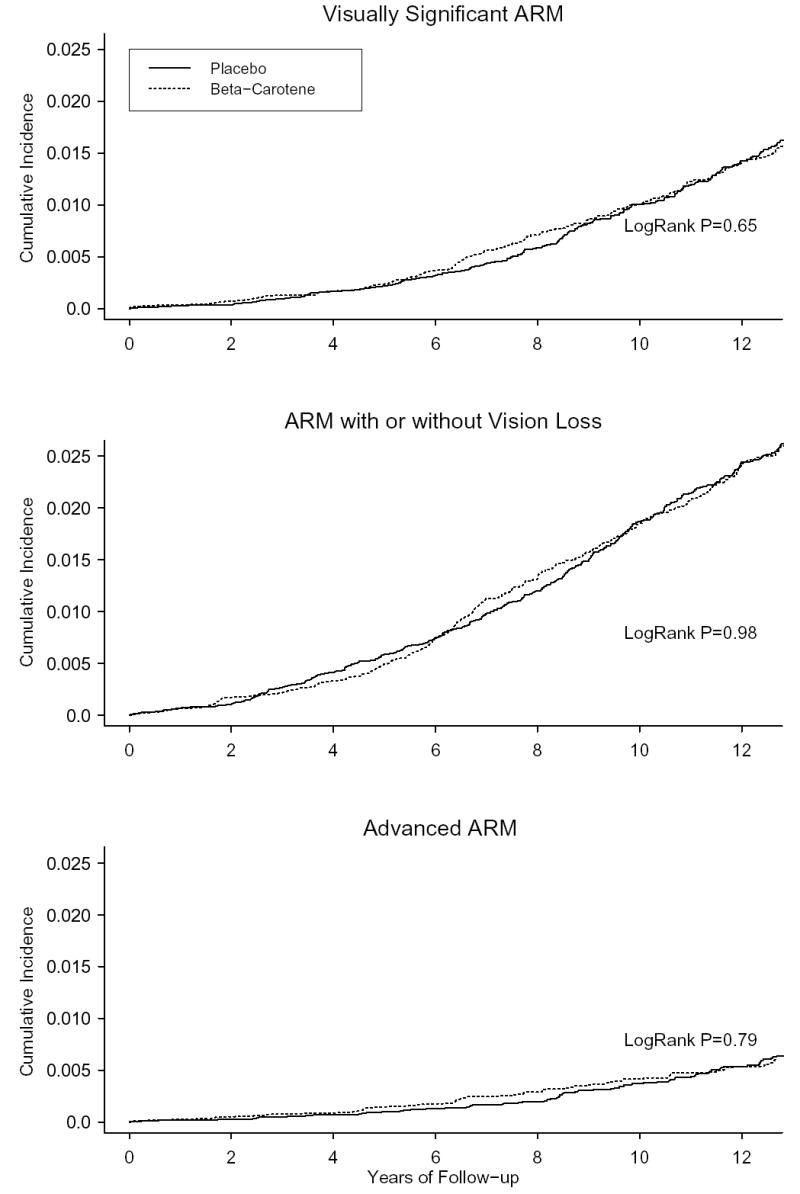
Cumulative incident rates of ARM.
There was no evidence for any modification of the lack of effect of beta carotene on ARM by baseline categories of cigarette smoking, alcohol use, body mass index, blood pressure, cholesterol, or multivitamin use. The results of these analyses for the endpoint of visually-significant ARM are shown in Table 3.
Table 3.
RRs and 95% CIs for Diagnosis of Visually-significant ARM According to Beta-Carotene Treatment Assignment, As Modified By Other Risk Factors, in Physicians’ Health Study I.
| No. of ARM/Total | ||||
|---|---|---|---|---|
| Beta-Carotene | Placebo | RR* (95% CI) | P Value (Interaction) | |
| Age | ||||
| 40–49 | 4/4479 | 0/4459 | --- | |
| 50–59 | 18/3620 | 29/3606 | 0.63 (0.35–1.13) | |
| 60–69 | 83/1900 | 77/1893 | 1.08 (0.80–1.48) | |
| 70–84 | 57/586 | 64/599 | 0.91 (0.63–1.30) | 0.94 |
| Smoke cigarettes | ||||
| Not-current | 137/9425 | 148/9411 | 0.94 (0.74–1.18) | |
| Current | 24/1132 | 21/1113 | 1.14 (0.63–2.05) | 0.58 |
| Alcohol use | ||||
| Daily | 55/2553 | 59/2572 | 0.90 (0.63–1.30) | |
| Weekly | 64/5261 | 65/5139 | 0.99 (0.70–1.40) | |
| Rarely | 42/2683 | 46/2760 | 0.99 (0.65–1.51) | 0.72 |
| Body mass index | ||||
| <25.0 | 84/6078 | 93/6093 | 0.90 (0.67–1.20) | |
| 25.0–29.9 | 70/4047 | 70/4047 | 1.06 (0.76–1.48) | |
| ≥ 30.0 | 8/455 | 7/417 | 1.07 (0.39–2.95) | 0.46 |
| Hypertension | ||||
| No | 87/8092 | 101/8042 | 0.80 (0.60–1.07) | |
| Yes | 72/2389 | 68/2420 | 1.17 (0.84–1.64) | 0.08 |
| High cholesterol level | ||||
| No | 119/8176 | 132/8133 | 0.91 (0.71–1.17) | |
| Yes | 27/1075 | 26/1137 | 1.17 (0.68–2.01) | 0.39 |
| Multivitamin use (current) | ||||
| No | 120/8530 | 124/8437 | 0.95 (0.74–1.22) | |
| Yes | 41/2019 | 46/2084 | 0.95 (0.63–1.45) | 0.97 |
Abbreviations: ARM, age-related maculopathy; RR, relative risk; CI, confidence interval.
Adjusted for age and aspirin treatment assignment
Discussion
These randomized trial data of 12 years duration from a large population of apparently healthy male physicians indicate that beta carotene treatment has no statistically significant beneficial or harmful effect on risk of ARM. Men in the beta carotene group had a non-significant 4% lower risk of the primary study endpoint of visually-significant ARM. The 95% CI around this estimate excluded a beneficial effect of 25% or greater or a harmful effect of 20% or greater. RR estimates for the secondary endpoints of ARM with or without vision loss and advanced ARM were also near the null value of 1.0.
Some potential limitations of the trial need to be considered, particularly in view of the null findings. It seems unlikely that an inadequate dosage of beta carotene explains the null findings. The dose used in this study (50 mg on alternate days) increased plasma beta carotene concentration approximately fourfold (18, 19), (similar to the increase in beta carotene concentration observed in AREDS [13]), and placed men in the beta carotene group in the top few percentiles of the general population with respect to usual intake. It also seems unlikely that the duration of treatment of 12 years was insufficient to materially reduce risks of ARM. In AREDS, the beneficial effect of high-dose antioxidants and zinc was apparent after a treatment duration of 6.3 years suggesting that the 12 years of treatment in PHS I was sufficient to detect any possible benefit of beta carotene. Exclusion of cases diagnosed during the first 2 years or first 5 years of follow-up had little impact on RR estimates suggesting there was no delayed effect for beta carotene. Poor compliance with assigned treatment also seems an unlikely explanation for our findings. At the end of the trial, compliance was still 78 percent in the beta carotene group, and only 6 percent in the placebo group reported the use of beta carotene or vitamin A supplements. Other possible explanations for the trial’s findings include bias in the ascertainment of the ARM endpoint and confounding by other factors. Because it was not feasible to perform ocular examinations on all participants, ascertainment of ARM cases was based on participant reports and thus some degree of underascertainment of ARM is plausible. However, underascertainment of disease is not associated with bias in randomized comparisons. Random misclassification of reported ARM, which would tend to shift the relative risk estimate toward the null, was reduced by the use of medical records to confirm the participant reports. Further, the participants are all physicians. Non-random or differential misclassification was unlikely since medical records were reviewed without knowledge of beta carotene treatment assignment, and study participants and treating ophthalmologists and optometrists were unaware of beta carotene treatment assignment. Confounding is unlikely in this large randomized trial since, as expected, baseline characteristics were equally distributed between the beta carotene and placebo groups. This equal distribution of risk factors provides reassurance that other potential confounders which were either unmeasured or unknown were also likely to be evenly distributed between the two treatment groups.
Beta carotene supplementation in ARM has been examined in two previous randomized trials. In the Alpha-Tocopherol Beta-Carotene (ATBC) study, a randomized trial of alpha-tocopherol (50 mg daily) and beta carotene (20 mg daily) conducted among more than 29,000 Finnish male smokers aged 50 to 69 years, end-of-trial eye examinations were conducted for a small sub-sample of 941 participants aged 65 years or more (20). Based on analysis of fundus photographs, 269 (29%) participants were judged to show signs of ARM at trial end with the majority of cases (239/269 [89%]) classified as dry maculopathy with hard drusen and/or pigmentary changes. There was no beneficial effect of beta carotene (RR, 1.04; 95% CI, 0.74–1.47) on the prevalence of ARM in that trial. In the other trial, AREDS examined whether daily supplements of zinc and an antioxidant combination of beta-carotene (15 mg), vitamin C (500 mg), and vitamin E (400 IU) could delay progression to advanced AMD in 3,640 participants aged 55–80 years at high risk (13). During an average of 6.3 years of follow-up, 803 incident cases of advanced AMD were identified. Compared to individuals assigned to placebo, those assigned to zinc plus antioxidants had a statistically significant 28 percent reduced risk of advanced AMD (RR, 0.72; 99% CI, 0.52–0.98). Those assigned to antioxidants alone had a somewhat smaller, and statistically non-significant, 20 percent reduced risk of advanced AMD (RR, 0.80; 99% CI, 0.59–1.09). Because the antioxidant agents were tested in combination, the specific effects of beta carotene, vitamin C, and vitamin E could not be determined in AREDS.
Our findings in PHS I are in general agreement with the findings of the ATBC study in suggesting that long-term supplemention with beta carotene has no marked beneficial or harmful effect on early stages of ARM development. Comparison of our findings with those of AREDS, however, is complicated by the absence of specific information on the effect of beta carotene in the AREDS formulation, and by the testing of a higher-risk population and use of a more severe ARM endpoint in that trial. When we considered only cases of ARM characterized by geographic atrophy or signs of exudative disease (advanced ARM, n=129), an endpoint more comparable to the endpoint of advanced AMD examined in AREDS, we found no evidence of benefit or harm for the 12 years of beta carotene treatment (RR, 0.97; 95% CI, 0.69–1.37). However, the 95% CI around this estimate was wide, probably owing to the smaller number of cases.
Initial enthusiasm for supplementation with beta carotene was tempered by the emergence of evidence in the mid 1990’s raising the possibility that large doses of beta carotene could be harmful in smokers (21, 22). However, the main trial results for PHS I indicated that 12 years of randomized beta carotene treatment had no beneficial or harmful effect on any cancer or cardiovascular disease endpoint in the overall population, or in the 11 percent of physicians who were current smokers at baseline (16). Two other randomized trials also reported no effect of beta carotene supplementation on cancer, cardiovascular, or mortality endpoints (23, 24).
Concern for the possible adverse effects of beta carotene in smokers caused some ophthalmologists to recommend that smokers avoid taking beta carotene in the AREDS formulation, and instead supplement with only some of the trial ingredients (15). Indeed, several additional preparations of the original AREDS supplement are already commercially available (15). It should be noted, however, that the effect of removing one or more ingredients from the AREDS formulation cannot be predicted and the impact on ARM of different combinations of antioxidant nutrients and other preventive agents will need to be tested in randomized trials. Our data, particularly as they apply to advanced ARM, suggest that the reduction in ARM progression observed for the antioxidant combination in AREDS is unlikely to reflect an important independent beneficial effect for beta carotene, and that removal of beta carotene from the AREDS formulation should have no material impact on the supplement’s efficacy. The observation that beta carotene is found only in trace amounts in the retina (25–28) provides some indirect support for this possibility. Nevertheless, a possible indirect effect of beta carotene in the AREDS formulation, mediated through interaction with other trial ingredients (29–31), cannot be excluded.
In summary, the results of this trial indicate that beta carotene supplementation for 12 years has little impact on the development of visually-significant ARM in apparently healthy men. These findings, together with forthcoming data from other completed and ongoing trials (32–35), will complement the findings in AREDS by clarifying the effects of individual antioxidants and other nutrient combinations, and should help assure rational clinical and public health recommendations for the prevention of ARM.
Acknowledgments
The authors would like to acknowledge the crucial contributions of the entire staff of the PHS, under the leadership of Charlene Belanger, as well as Mary Breen, Vadim Bubes, Jean MacFadyen, Geneva McNair, David Potter, Leslie Power, Harriet Samuelson, Miriam Schvartz, Mickie Sheehey, Joanne Smith and Phyllis Johnson Wojciechowski. We are also indebted to the 22,071 dedicated and committed participants of the PHS. Dr. Christen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Supported by research grants HL 26490, HL 34595, CA 34944, CA 40360, and EY 06633 from the National Institutes of Health.
References
- 1.National Advisory Eye Council. Bethesda, Maryland: 2004. National Plan for Eye and Vision Research. NIH Publication No. 04–4288. [Google Scholar]
- 2.Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 3.Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials Arch Ophthalmol. 1991;109:1109–1114. [PubMed] [Google Scholar]
- 4.Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials Arch Ophthalmol. 1993;111:1200–1209. doi: 10.1001/archopht.1993.01090090052019. [DOI] [PubMed] [Google Scholar]
- 5.Bressler NM Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol. 2001;119:198–207. [PubMed] [Google Scholar]
- 6.Verteporfin In Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization--verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131:541–560. doi: 10.1016/s0002-9394(01)00967-9. [DOI] [PubMed] [Google Scholar]
- 7.Gragoudas ES, Adamis AP, Cunningham ET, Jr, Feinsod M, Guyer DR. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–2816. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 8.Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–1047. doi: 10.1016/j.ophtha.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Fekrat S, Bressler SB. Are antioxidants or other supplements protective for age-related macular degeneration? Curr Opin Ophthalmol. 1996;7:65–72. doi: 10.1097/00055735-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Moeller SM, Jacques PF, Blumberg JB. The potential role of dietary xanthophylls in cataract and age-related macular degeneration. J Am Coll Nutr. 2000;19:522S–527S. doi: 10.1080/07315724.2000.10718975. [DOI] [PubMed] [Google Scholar]
- 11.Mares-Perlman JA, Millen AE, Ficek TL, Hankinson SE. The body of evidence to support a protective role for lutein and zeaxanthin in delaying chronic disease. Overview J Nutr. 2002;132:518S–524S. doi: 10.1093/jn/132.3.518S. [DOI] [PubMed] [Google Scholar]
- 12.Hogg R, Chakravarthy U. AMD and micronutrient antioxidants. Curr Eye Res. 2004;29:387–401. doi: 10.1080/02713680490517890. [DOI] [PubMed] [Google Scholar]
- 13.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8 Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jampol LM, Ferris FL., 3rd Antioxidants and zinc to prevent progression of age-related macular degeneration. JAMA. 2001;286:2466–2468. [PubMed] [Google Scholar]
- Jampol LM. AREDS--two years later. Arch Ophthalmol. 2003;121:1634–1636. doi: 10.1001/archopht.121.11.1634. [DOI] [PubMed] [Google Scholar]
- 16.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 17.The Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 18.Satterfield S, Greco PJ, Goldhaber SZ, et al. Biochemical markers of compliance in the Physicians’ Health Study. Am J Prev Med. 1990;6:290–294. [PubMed] [Google Scholar]
- 19.Fotouhi N, Meydani M, Santos MS, Meydani SN, Hennekens CH, Gaziano JM. Carotenoid and tocopherol concentrations in plasma, peripheral blood mononuclear cells, and red blood cells after long-term beta-carotene supplementation in men. Am J Clin Nutr. 1996;63:553–558. doi: 10.1093/ajcn/63.4.553. [DOI] [PubMed] [Google Scholar]
- 20.Teikari JM, Laatikainen L, Virtamo J, et al. Six-year supplementation with alpha-tocopherol and beta-carotene and age-related maculopathy. Acta Ophthalmol Scand. 1998;76:224–229. doi: 10.1034/j.1600-0420.1998.760220.x. [DOI] [PubMed] [Google Scholar]
- 21.Goodman GE, Thornquist MD, Balmes J, et al. The Beta-Carotene and Retinol Efficacy Trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 2004;96:1743–17450. doi: 10.1093/jnci/djh320. [DOI] [PubMed] [Google Scholar]
- 22.Virtamo J, Pietinen P, Huttunen JK, et al. Incidence of cancer and mortality following alpha-tocopherol and beta-carotene supplementation: a postintervention follow-up. JAMA. 2003;290:476–485. doi: 10.1001/jama.290.4.476. [DOI] [PubMed] [Google Scholar]
- 23.Greenberg ER, Baron JA, Karagas MR, et al. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA. 1996;275:699–703. doi: 10.1001/jama.1996.03530330043027. [DOI] [PubMed] [Google Scholar]
- 24.Lee IM, Cook NR, Manson JE, Buring JE, Hennekens CH. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women’s Health Study. J Natl Cancer Inst. 1999;91:2102–2106. doi: 10.1093/jnci/91.24.2102. [DOI] [PubMed] [Google Scholar]
- Bone RA, Landrum JT, Fernandez L, Tarsis SL. Analysis of the macular pigment by HPLC: retinal distribution and age study. Invest Ophthalmol Vis Sci. 1988;29:843–849. [PubMed] [Google Scholar]
- 26.Handelman GJ, Dratz EA, Reay CC, van Kuijk FJGM. Carotenoids in the human macula and whole retina. Invest Ophthalmol Vis Sci. 1988;29:850–855. [PubMed] [Google Scholar]
- 27.Bernstein PS, Khachik F, Carvalho LS, Muir GJ, Zhao DY, Katz NB. Identification and quantitation of carotenoids and their metabolites in the tissues of the human eye. Exp Eye Res. 2001;72:215–223. doi: 10.1006/exer.2000.0954. [DOI] [PubMed] [Google Scholar]
- 28.Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385:28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- 29.Niki E, Noguchi N, Tsuchihashi H, Gotoh N. Interaction among vitamin C, vitamin E, and beta-carotene. Am J Clin Nutr. 1995;62:1322S–1326S. doi: 10.1093/ajcn/62.6.1322S. [DOI] [PubMed] [Google Scholar]
- 30.Bohm F, Edge R, McGarvey DJ, Truscott TG. Beta-carotene with vitamins E and C offers synergistic cell protection against Nox. FEBS Lett. 1998;436:387–389. doi: 10.1016/s0014-5793(98)01173-9. [DOI] [PubMed] [Google Scholar]
- 31.Young AJ, Lowe GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys. 2001;385:20–27. doi: 10.1006/abbi.2000.2149. [DOI] [PubMed] [Google Scholar]
- 32.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer. The Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 33.Manson JE, Gaziano JM, Spelsberg A, et al. for the WACS Research Group. A secondary prevention trial of antioxidant vitamins and cardiovascular disease in women: rationale, design, and methods. Ann Epidemiol. 1995;5:261–269. doi: 10.1016/1047-2797(94)00091-7. [DOI] [PubMed] [Google Scholar]
- 34.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II--a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125–134. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 35.Lippman SM, Goodman PJ, Klein EA, et al. Designing the Selenium and Vitamin E Cancer Prevention Trial (SELECT) J Natl Cancer Inst. 2005;97:94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]


