Abstract
Opiates and cocaine both have effects on adrenal and gonadal function. Opiates suppress the hypothalamic-pituitary adrenal (HPA) axis, whereas cocaine leads to HPA activation. Opiates also cause gonadal dysfunction in both men and women. During withdrawal from opiates and cocaine, the HPA axis is activated which may reinforce relapse behavior. This review describes these hormonal effects and explores the potential consequences, including the effects on mood cognition and cardiovascular risk. Modification of the drug-induced hormonal dysfunction may represent a treatment strategy for drug rehabilitation.
Keywords: Gonadal and adrenal abnormalities, drug users, poor health outcomes, cardiovascular risk
INTRODUCTION
Opiates and cocaine are considered to be the main problem drugs of abuse worldwide[1]. In the United States, 2.3 million are current cocaine users and an estimated 119,000 abuse heroin according to a recent national survey[2]. Abuse of both of these drugs is associated with multiple poor health outcomes, reduced quality of life and criminal behavior.
Opiates and cocaine both have important physiologic effects on multiple organ systems. In the endocrine system, both of these drugs can have immediate and potentially long-term effects on the hypothalamic-pituitary-adrenal (HPA) and the hypothalamic-pituitary-gonadal (HPG) axes. The consequences of these changes are unclear. By affecting mood, stress, cognition and energy, these drugs may lead to or potentiate depressive symptomatology and reduced quality of life. In addition, changes in gonadal and adrenal function may induce changes in body composition, which could increase cardiovascular risk. During drug withdrawal, some studies have shown activation of the HPA axis, possibly contributing to relapse behavior.
This review will focus on the extent to which the HPG and HPA axes are altered with exposure to opiates and cocaine and the potential for these changes to lead to depression, reduced quality of life and illness. Given the high prevalence of HIV infection in drug users, possible interactions between drugs of abuse, HIV infection and antiretroviral treatment will also be considered. Drug abusers are a vulnerable population. To optimize drug abuse treatment, a full understanding of the physiologic, psychological and social issues is required. The endocrine abnormalities present in drug users may be a potential target for drug abuse treatment and prevention.
Gonadal abnormalities in drug use
Normal gonadal function requires intact communication between the hypothalamus, the pituitary and the testes in males and the ovaries in females. Gonadotropin releasing hormone (GnRH) is secreted in a pulsatile fashion from the preoptic area and the medial basal region of the hypothalamus, inducing secretion of leutinizing hormone (LH) and follicle stimulating hormone (FSH) from the anterior pituitary. In males, LH stimulates testosterone synthesis in the Leydig cells of the testes and FSH induces spermatogenesis in Sertoli cells. In females, FSH causes maturation of an ovarian follicle and LH induces ovulation.
GnRH secretion is partially regulated by endogenous opioids, such as β-endorphins, which produce tonic inhibition of its release[3]. Exogenously administered opioids also inhibit GnRH secretion, resulting in HPG dysfunction at the level of the hypothalamus. In experimental models, methadone administration was found to significantly reduce testosterone concentrations by over two-thirds of the baseline values after 60 minutes, mediated in part by the inhibition of dopaminergic activity in the hypothalamus[4]. In addition to their effects on the hypothalamus, opioids have also been shown to directly reduce testosterone synthesis[5].
Opiate-induced effects on HPG axis integrity can lead to clinical hypogonadism. In a study of detoxified heroin addicts, intravenous administration of heroin (10 mg) produced acute suppression of LH secretion, leading to reduced testosterone concentration with a peak effect after 4–6 hours[6]. In accord with these experimental findings, multiple cross-sectional studies in heroin dependent subjects have demonstrated decreased gonadotropin and testosterone levels[7–11], which can be reversed with the administration of opiate antagonists[12]. This latter observation suggests a direct effect of heroin on the HPG axis, independent of the effect of potential confounding factors, such as poor nutrition, alcohol abuse and chronic illness. Reduced testosterone is also seen commonly among those on methadone maintenance[13]. In addition, opiate administration is also associated with abnormal spermatogenesis in men[14] and has been associated with reduced sexual performance. In females, opiate administration leads to significant inhibition of LH release. This effect on gonadotropin control likely contributes to the 50% prevalence of menstrual dysfunction observed in female heroin users[15].
Cocaine exposure can also disrupt normal gonadal function. In an animal model of cocaine addiction, binge cocaine administration to rats caused decreased testosterone concentrations[16]. In men, acute cocaine administration is associated with HPG axis dysregulation, with increases of LH of unclear significance[17]. In females, both clinical and animal studies have also shown disruption of the menstrual cycle with cocaine administration probably through its effects on LH release[18].
Adrenal dysfunction in drug use
In healthy individuals, pulsatile secretion of corticotropin releasing hormone (CRH) in the hypothalamus causes pituitary secretion of adrenocorticotropic hormone (ACTH), which stimulates cortisol synthesis and release from the adrenal cortex. Opiate administration disrupts this pathway through the inhibition of CRH release, leading to reduced cortisol production[19]. Consistent with this observation, small studies have shown a high prevalence of adrenal insufficiency in opiate dependent subjects, with a high prevalence of abnormal ACTH stimulation tests[20], low cortisol concentrations accompanied by low ACTH levels and atypical circadian production of these hormones[21]. In addition, the administration of metyrapone, a cortisol synthesis inhibitor used to test the integrity of the HPA axis, shows decreased HPA activation in heroin users[22]. An attenuated response to CRH in methadone users compared to non-users suggests that disruption of the HPA axis is at the level of the pituitary or adrenal gland.
In contrast to opiate use, cocaine administration results in enhanced HPA activity in both humans[23] and non-human primate species[24]. Interestingly, male monkeys have a more robust HPA response to cocaine use compared to females[24] however in rats, females exhibit a greater HPA response to cocaine than males. CRH antagonists block hypercortisolism observed in cocaine users across species studied[16]. However, the administration of metyrapone increased HPA activity in cocaine users, illustrating different effects of opiates and stimulants on HPA function.
Potential consequences of adrenal and gonadal abnormalities
Depression, stress, quality of life and cognition
Hypogonadism and adrenal dysfunction can have important effects on mood and cognition. In men with hypogonadism, depressive symptoms are common[25]. Although the mechanism has not been fully clarified, animal models suggest that testosterone and estrogen have direct effects on central serotonergic receptors[26]. In several populations with prevalent hypogonadism, including aging males and HIV-infected men, depressive symptoms, including sadness and anger can be reversed with the restoration of normal androgen levels[27,28].
Reduced cortisol concentrations have also been linked to depressive symptoms. Twenty to forty percent of patients with adrenal insufficiency have depression, manifesting itself as apathy and profound fatigue, sometimes associated with delusional behavior. Subtle mood alterations may be the initial symptom in patients with adrenal insufficiency[29]. Major depression has been reported in 34% of drug users[30], with moderate to severe depressive symptoms reports in over half[31]. Mood abnormalities can have important repercussions on drug abuse behavior, in that drug dependent individuals with depression are more like to be unsuccessful in attempts to abstain[32]. In addition, the presence of major depression predicts relapse in abstinent patients[33]. The extent to which depressive symptoms in drug users are related to abnormalities in the HPG or HPA axes has not been clarified.
Major depression and psychological stress cause activation of the HPA axis[34]. With the high prevalence of depression and anxiety symptoms among drug users, psychological dysfunction may be another mechanism by which the HPA axis is activated in drug users. The consequences of this activation have not been fully explored, but these abnormalities may further contribute to metabolic alterations, leading to increased cardiovascular disease risk.
The cognitive consequences of drug abuse, as well as the cognitive impact of the associated risks such as HIV infection and endocrine pathologies that are observed with drug abuse, constitute an area that requires further study. Depression-related abnormalities in cortisol production are also correlated with memory dysfunction in individuals without HIV infection who are not administering opiates or stimulants. Additionally, there is a large literature concerning the modulatory effects of sex steroid hormones on cognition in people who are neither HIV-infected nor drug users. In vitro human neuronal cultures that include neurotoxic HIV proteins and exposed to a variety of stimulants such as cocaine were protected from the neurotoxic effects of the drugs by treatment with estrogen. Data are currently lacking regarding the potential impact of adrenal and gonadal hormone abnormalities and treatments on cognitive performance in drug abusing populations who may or may not be infected with HIV.
Metabolic complications
In addition to the important effects on mood, gonadal and adrenal dysfunction has repercussions on metabolism. Low testosterone concentrations cause reduced bone mineral density and alter body composition, leading to decreased lean body mass and increased fat mass. This latter effect, particularly when the fat accumulation is in the central compartment, is strongly related to insulin resistance and increased risk of cardiovascular disease[35]. Hypogonadism has also been associated with increased arterial stiffness, which may further contribute to cardiovascular risk[36].
Abnormal HPA dynamics in the drug user may also lead to metabolic alterations associated with increased cardiovascular risk. Hypercortisolemia, which is observed with cocaine administration and after the acute effects of opiates on the HPA axis have subsided, could also contribute to central adiposity and insulin resistance. In non-drug using populations, HPA axis activation is related to abdominal obesity and insulin resistance in both men and women[6,37]. Whether HPA and HPG dysregulation is involved in the development of cardiovascular risk factors in drug users is unknown.
HIV infection and treatment as a confounder
In the US, over 50% of new HIV infections occur in drug users. Direct viral effects or effects for antiretroviral medications result in a high prevalence of hypogonadism, altered concentrations of sex hormone binding globulins, altered circadian production of steroids, adrenal dysfunction and metabolic abnormalities in HIV infected men and women[38]. For this reason, the relationship between endocrine and metabolic abnormalities in drug users may be confounded by HIV infection and treatment. Alternatively, HIV-infection/treatment could act as an effect modifier, enhancing the impact of opiates and cocaine on endocrine and metabolic parameters in drug users. For this reason, epidemiological studies of endocrinology and metabolism in drug users should include a careful characterization of HIV status and treatment history, such that the effects of drugs of abuse and the effects of HIV and its treatment can be distinguished.
Adrenal and gonadal abnormalities in drug treatment
The hypothalamic-pituitary-adrenal axis also plays a critical role in craving and relapse. In animal models of addiction, experimental manipulation of the HPA axis can alter drug seeking behavior. When CRH is infused into rats previously exposed to heroin, drug-seeking behavior will be reinstated, particularly following physical stress. Conversely, blocking the effects of CRH, using a specific antagonist, attenuates a stress-induced relapse in the same model[39]. In a rat model of cocaine addiction, CRH also plays a synergistic role in stress-induced cocaine seeking behavior[40]. The mechanism underlying these observations is unclear, but glucocorticoids appear to sensitize the mesolimbic system, such that the dopaminergic reward pathway is hyperstimulated upon re-exposure to the drug[41]. Taken together, these experimental findings would suggest that HPA axis activation in cocaine and heroin abusers may lead to relapse behavior.
Indeed, gonadal and adrenal abnormalities are also seen in humans who discontinue opiate and cocaine administration. HPA axis dynamics in drug users shift when opiates are withdrawn. Without the suppressive effect of opiates on the HPA axis, the axis becomes hyperstimulated, resulting in hypercortisolism. This effect can be present 16 days after last opiate consumption[42] and has been attributed to increased release of CRH. Similarly, acute adminstration of cocaine activates the HPA axis, resulting in hypercortisolism[24,43]. In addition, HPA activation has been observed after cocaine cessation[23] and has been implicated in the pathophysiology of craving.
In human studies, HPA activation during drug use and between drug use episodes also reinforces craving and drug seeking-behavior. Methadone treated patients show withdrawal symptoms when exposed to metyrapone[44]. One interpretation of these data is that the resulting increases in CRH and ACTH after metyrapone may induce these negative effects, which then motivates the subject to seek drugs to alleviate these symptoms. Indeed, CRH has been shown to be important in the anxiogenic and adverse effects of drug withdrawal. In an animal model of opiate dependence, administration of a CRH antagonist reversed the conditioned aversion response elicited by an opiate antagonist[45].
Depression induced by hypogonadism may further lead to HPA activation and contribute to drug-seeking behavior. In addition, hypogonadism may contribute to craving by a more direct mechanism. Low levels of estrogen and testosterone reduce endogenous central nervous system opioid expression[46,47]. The resulting decreases in endogenous opioid levels during withdrawal and abstinence may be associated with craving for opioids and contribute to relapse.
Hormonal treatment of drug abuse
Given the potential role of HPA and HPG axis abnormalities in craving and relapse in drug dependent subjects, these endocrine systems may provide a target to improve drug treatment. In a rat model of cocaine addiction, pre-treatment with the cortisol synthesis inhibitor, ketoconazole, leads to a reduction in cocaine-seeking behavior, accompanied by lower plasma corticosteroid concentrations[48]. In humans, however, a randomized, placebo-controlled trial of ketoconazole in methadone maintained patients failed to show any benefit on illicit drug use behavior. In this trial of 39 subjects, those randomized to 600–900 mg/day of ketoconazole plus evening hydrocortisone (20 mg) were more likely to show evidence of cocaine self-administration compared to the placebo treated group. In contrast to prior animal studies, ketoconazole failed to suppress plasma cortisol concentrations. The reasons for these unexpected findings are unclear. It was postulated by the authors that patients randomized to ketoconazole may have been self-medicating to minimize the adverse effects of the study drug, including dyspepsia, nausea and body aches. In addition, the use of evening hydrocortisone may have affected the results by further altering HPA axis dynamics. Nevertheless, these results corroborate the findings of a lack of effect with acute administration of ketoconazole[49]. While ketoconazole does not appear to show promise as a potential drug treatment strategy, it is unknown whether other pharmacologic manipulation of the HPA axis will show any efficacy.
The HPG axis is another potential target for the treatment of drug abuse. In experimental animal models, hypogonadal rodents given androgen replacement were less sensitized to the behavioral effects of cocaine[50,51]. However, another animal model showed no effect of testosterone replacement on cocaine self-administration[52]. It is not clear whether the testosterone replacement in hypogonadal drug users will reduce craving and treatment failures.
CONCLUSION
The relationship between illicit drug use, abnormalities in gonadal and adrenal function and stress and depression is complex. Adrenal and gonadal dysfunction is both a cause and consequence of drug use behavior (see Fig. 1). There is, however, significant variation in the effect of opiates and cocaine on adrenal and gonadal function with both administration and withdrawal. Further, attempts to manipulate endocrine function in the treatment of addiction have had mixed results. It is possible that certain subsets of drug users are particularly vulnerable to the effects of opiates and cocaine on the HPG and HPA axes. Polymorphisms in the mu-opioid receptor, for example, have been associated with reduced HPA activation in the setting of receptor blockage with naloxone (Wand, 2002). Whether this genetic variation accounts for differences in the endocrine effects of drug administration and changes in endocrine function during withdrawal requires further study.
Fig. 1.
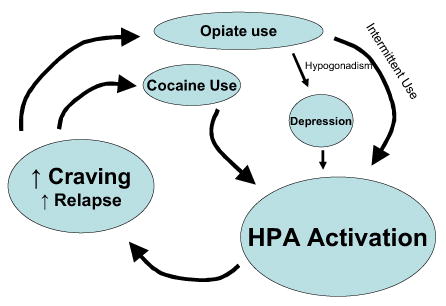
Conceptual Framework of the Hormonal Consequences of Drug Use. Cocaine and intermittent opiate exposure activate the hypothalamic-pituitary-adrenal (HPA) axis. Depression, which may be induced by opiate-related hypogondasim, also contributes to HPA activation. Changes in HPA dynamics lead to cravings and relapse behavior
The changes in gonadal and adrenal function induced by opiates and cocaine use may have effects on quality of life, mood and cardiovascular risk. Furthermore, alterations in these hormonal axes may be important in the perpetuation of drug use behavior and may serve as targets for improved drug treatment.
References
- 1.National Survey on Drug Use & Health. 2003 http://oas.samhsa.gov/nhsda/2k3nsduh/2k3Overview.htm#toc.
- 2.World Drug Report. 2004 http://www.unodc.org/unodc/en/world_drug_report.html.
- 3.Jenkins PJ, Grossman A. The control of the gonadotrophin releasing hormone pulse generator in relation to opioid and nutritional cues. Hum Reprod. 1993;8(Suppl 2):154–161. doi: 10.1093/humrep/8.suppl_2.154. [DOI] [PubMed] [Google Scholar]
- 4.Singh HH, Purohit V, Ahluwalia BS. Methadone blocks dopamine-mediated release of gonadotropins in rat hypothalamus. Neuroendocrinology. 1982;34:347–352. doi: 10.1159/000123326. [DOI] [PubMed] [Google Scholar]
- 5.Adams ML, Sewing B, Forman JB, Meyer ER, Cicero TJ. Opioid-induced suppression of rat testicular function. J Pharmacol Exp Ther. 1993;266:323–328. [PubMed] [Google Scholar]
- 6.Mirin SM, Meyer RE, Mendelson JH, Ellingboe J. Opiate use and sexual function. Am J Psychiat. 1980;37:909–915. doi: 10.1176/ajp.137.8.909. [DOI] [PubMed] [Google Scholar]
- 7.Brambilla F, Sacchetti E, Brunetta M. Pituitary-gonadal function in heroin addicts. Neuropsychobiology. 1977;3:160–166. doi: 10.1159/000117601. [DOI] [PubMed] [Google Scholar]
- 8.Celani MF, Carani C, Montanini V, Baraghini GF, Zini D, Simoni M, Ferretti C, Marrama P. Further studies on the effects of heroin addiction on the hypothalamic-pituitary-gonadal function in man. Pharmacol Res Commun. 1984;16:1193–1203. doi: 10.1016/s0031-6989(84)80084-3. [DOI] [PubMed] [Google Scholar]
- 9.Mendelson JH, Meyer RE, Ellingboe J, Mirin SM, McDougle M. Effects of heroin and methadone on plasma cortisol and testosterone. J Pharmacol Exp Ther. 1975a;195:296–302. [PubMed] [Google Scholar]
- 10.Mendelson JH, Ellingboe J, Kuehnle JC, Mello NK. Heroin and naltrexone effects on pituitary-gonadal hormones in man: interaction of steroid feedback effects, tolerance and supersensitivity. J Pharmacol Exp Ther. 1980b;214:503–506. [PubMed] [Google Scholar]
- 11.Wang C, Chan V, Yeung RT. The effect of heroin addiction on pituitary-testicular function. Clin Endocrinol (Oxf) 1978;9:455–461. doi: 10.1111/j.1365-2265.1978.tb03585.x. [DOI] [PubMed] [Google Scholar]
- 12.Mendelson JH, Ellingboe J, Kuehnle JC, Mello NK. Heroin and naltrexone effects on pituitary-gonadal hormones in man: interaction of steroid feedback effects, tolerance and supersensitivity. J Pharmacol Exp Ther. 1980a;214:503–506. [PubMed] [Google Scholar]
- 13.Mendelson JH, Meyer RE, Ellingboe J, Mirin SM, McDougle M. Effects of heroin and methadone on plasma cortisol and testosterone. J Pharmacol Exp Ther. 1975b;195:296–302. [PubMed] [Google Scholar]
- 14.Ragni G, De Lauretis L, Gambaro V, Di Pietro R, Bestetti O, Recalcati F, Papetti C. Semen evaluation in heroin and methadone addicts. Acta Eur Fertil. 1985;16:245–249. [PubMed] [Google Scholar]
- 15.Santen FJ, Sofsky J, Bilic N, Lippert R. Mechanism of action of narcotics in the production of menstrual dysfunction in women. Fertil Steril. 1975;26:538–548. [PubMed] [Google Scholar]
- 16.Sarnyai Z, Dhabhar FS, McEwen BS, Kreek MJ. Neuroendocrine-related effects of long-term, 'binge' cocaine administration: diminished individual differences in stress-induced corticosterone response. Neuroendocrinology. 1998;68:334–344. doi: 10.1159/000054382. [DOI] [PubMed] [Google Scholar]
- 17.Mendelson JH, Sholar MB, Siegel AJ, Mello NK. Effects of cocaine on luteinizing hormone in women during the follicular and luteal phases of the menstrual cycle and in men. J Pharmacol Exp Ther. 2001;296:972–979. [PubMed] [Google Scholar]
- 18.Mello NK, Mendelson JH. Cocaine's effects on neuroendocrine systems: clinical and preclinical studies. Pharmacol Biochem Behav. 1997;57:571–599. doi: 10.1016/s0091-3057(96)00433-9. [DOI] [PubMed] [Google Scholar]
- 19.Auernhammer CJ, Renner U, Muller OA, Stalla J, Stalla GK. Loperamide inhibits corticotrophic cell function by a naloxone-insensitive mechanism in the rat in vitro. Neuroendocrinology. 1993;57:1019–1027. doi: 10.1159/000126466. [DOI] [PubMed] [Google Scholar]
- 20.Tennant F, Shannon JA, Nork JG, Sagherian A, Berman M. Abnormal adrenal gland metabolism in opioid addicts: implications for clinical treatment. J Psychoactive Drugs. 1991;23:135–149. doi: 10.1080/02791072.1991.10472232. [DOI] [PubMed] [Google Scholar]
- 21.Facchinetti F, Grasso A, Petraglia F, Parrini D, Volpe A, Genazzani AR. Impaired circadian rhythmicity of beta-lipotrophin, beta-endorphin and ACTH in heroin addicts. Acta Endocrinol (Copenh) 1984;105:149–155. doi: 10.1530/acta.0.1050149. [DOI] [PubMed] [Google Scholar]
- 22.Cushman P, Jr, Kreek MJ. Some endocrinologic observations in drug addicts. 1994:161–173. [Google Scholar]
- 23.Contoreggi C, Herning RI, Koeppl B, Simpson PM, Negro PJ, Jr, Fortner-Burton C, Hess J. Treatment-seeking inpatient cocaine abusers show hypothalamic dysregulation of both basal prolactin and cortisol secretion. Neuroendocrinology. 2003;78:154–162. doi: 10.1159/000072797. [DOI] [PubMed] [Google Scholar]
- 24.Broadbear JH, Winger G, Cicero TJ, Woods JH. Effects of self-administered cocaine on plasma adrenocorticotropic hormone and cortisol in male rhesus monkeys. J Pharmacol Exp Ther. 1999;289:1641–1647. [PubMed] [Google Scholar]
- 25.Burris AS, Banks SM, Carter CS, Davidson JM, Sherins RJ. A long-term, prospective study of the physiologic and behavioral effects of hormone replacement in untreated hypogonadal men. J Androl. 1992;13:297–304. [PubMed] [Google Scholar]
- 26.Sumner BE, Fink G. Testosterone as well as estrogen increases serotonin2A receptor mRNA and binding site densities in the male rat brain. Brain Res Mol Brain Res. 1998;59:205–214. doi: 10.1016/s0169-328x(98)00148-x. [DOI] [PubMed] [Google Scholar]
- 27.Dobs A. Role of testosterone in maintaining lean body mass and bone density in HIV-infected patients. Intl J Impot Res. 2003;15(Suppl 4):S21–S25. doi: 10.1038/sj.ijir.3901032. [DOI] [PubMed] [Google Scholar]
- 28.Wang C, Alexander G, Berman N, Salehian B, Davidson T, McDonald V, Steiner B, Hull L, Callegari C, Swerdloff RS. Testosterone replacement therapy improves mood in hypogonadal men--a clinical research center study. J Clin Endocrinol Metab. 1996;81:3578–3583. doi: 10.1210/jcem.81.10.8855804. [DOI] [PubMed] [Google Scholar]
- 29.Nieman LK, Orth DN. Clinical manifestations of adrenal insufficiency. 2004. [Google Scholar]
- 30.Dinwiddie SH, Reich T, Cloninger CR. Psychiatric comorbidity and suicidality among intravenous drug users. J Clin Psychiat. 1992;53:364–369. [PubMed] [Google Scholar]
- 31.Golub ET, Latka M, Hagan H, Havens JR, Hudson SM, Kapadia F, Campbell JV, Garfein RS, Thomas DL, Strathdee SA. Screening for depressive symptoms among HCV-infected injection drug users: examination of the utility of the CES-D and the beck depression inventory. J Urban Health. 2004;81:278–290. doi: 10.1093/jurban/jth114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasin D, Liu X, Nunes E, McCloud S, Samet S, Endicott J. Effects of major depression on remission and relapse of substance dependence. Arch Gen Psychiat. 2002b;59:375–380. doi: 10.1001/archpsyc.59.4.375. [DOI] [PubMed] [Google Scholar]
- 33.Hasin D, Liu X, Nunes E, McCloud S, Samet S, Endicott J. Effects of major depression on remission and relapse of substance dependence. Arch Gen Psychiat. 2002a;59:375–380. doi: 10.1001/archpsyc.59.4.375. [DOI] [PubMed] [Google Scholar]
- 34.Parker KJ, Schatzberg AF, Lyons DM. Neuroendocrine aspects of hypercortisolism in major depression. Horm Behav. 2003;43:60–66. doi: 10.1016/s0018-506x(02)00016-8. [DOI] [PubMed] [Google Scholar]
- 35.Bhasin S. Effects of testosterone administration on fat distribution, insulin sensitivity and atherosclerosis progression. Clin Infect Dis. 2003;37(Suppl 2):S142–S149. doi: 10.1086/375878. [DOI] [PubMed] [Google Scholar]
- 36.Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, Mason MD, Cockcroft JR, Scanlon MF, Davies JS. The effects of induced hypogonadism on arterial stiffness, body composition and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–4267. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 37.Pasquali R, Cantobelli S, Casimirri F, Capelli M, Bortoluzzi L, Flamia R, Labate AM, Barbara L. The hypothalamic-pituitary-adrenal axis in obese women with different patterns of body fat distribution. J Clin Endocrinol Metab. 1993;77:341–346. doi: 10.1210/jcem.77.2.8393881. [DOI] [PubMed] [Google Scholar]
- 38.Cooper OB, Brown TT, Dobs AS. Opiate drug use: a potential contributor to the endocrine and metabolic complications in human immunodeficiency virus disease. Clin Infect Dis. 2003;37(Suppl 2):S132–S136. doi: 10.1086/375879. [DOI] [PubMed] [Google Scholar]
- 39.Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- 42.Cami J, Gilabert M, San L, de la TR. Hypercortisolism after opioid discontinuation in rapid detoxification of heroin addicts. Br J Addict. 1992;87:1145–1151. doi: 10.1111/j.1360-0443.1992.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 43.Heesch CM, Negus BH, Keffer JH, Snyder RW, Risser RC, Eichhorn EJ. Effects of cocaine on cortisol secretion in humans. Am J Med Sci. 1995;310:61–64. doi: 10.1097/00000441-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Kennedy JA, Hartman N, Sbriglio R, Khuri E, Kreek MJ. Metyrapone-induced withdrawal symptoms. Br J Addict. 1990;85:1133–1140. doi: 10.1111/j.1360-0443.1990.tb03438.x. [DOI] [PubMed] [Google Scholar]
- 45.Heinrichs SC, Menzaghi F, Schulteis G, Koob GF, Stinus L. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995;6:74–80. [PubMed] [Google Scholar]
- 46.Adams LA, Vician L, Clifton DK, Steiner RA. Testosterone regulates pro-opiomelanocortin gene expression in the primate brain. Endocrinology. 1991;128:1881–1886. doi: 10.1210/endo-128-4-1881. [DOI] [PubMed] [Google Scholar]
- 47.Matera C, Wardlaw SL. Aromatization is not required for androgen induced changes in proopiomelanocortin gene expression in the hypothalamus. Brain Res Mol Brain Res. 1994;27:275–280. doi: 10.1016/0169-328x(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 48.Goeders NE, Peltier RL, Guerin GF. Ketoconazole reduces low dose cocaine self-administration in rats. Drug Alcohol Depend. 1998;53:67–77. doi: 10.1016/s0376-8716(98)00108-2. [DOI] [PubMed] [Google Scholar]
- 49.Ward AS, Collins ED, Haney M, Foltin RW, Fischman MW. Ketoconazole attenuates the cortisol response but not the subjective effects of smoked cocaine in humans. Behav Pharmacol. 1998;9:577–586. doi: 10.1097/00008877-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 50.Chen R, Osterhaus G, McKerchar T, Fowler SC. The role of exogenous testosterone in cocaine-induced behavioral sensitization and plasmalemmal or vesicular dopamine uptake in castrated rats. Neurosci Lett. 2003;351:161–164. doi: 10.1016/j.neulet.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 51.Long SF, Dennis LA, Russell RK, Benson KA, Wilson MC. Testosterone implantation reduces the motor effects of cocaine. Behav Pharmacol. 1994;5:103–106. doi: 10.1097/00008877-199402000-00012. [DOI] [PubMed] [Google Scholar]
- 52.Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29:929–942. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]


