Abstract
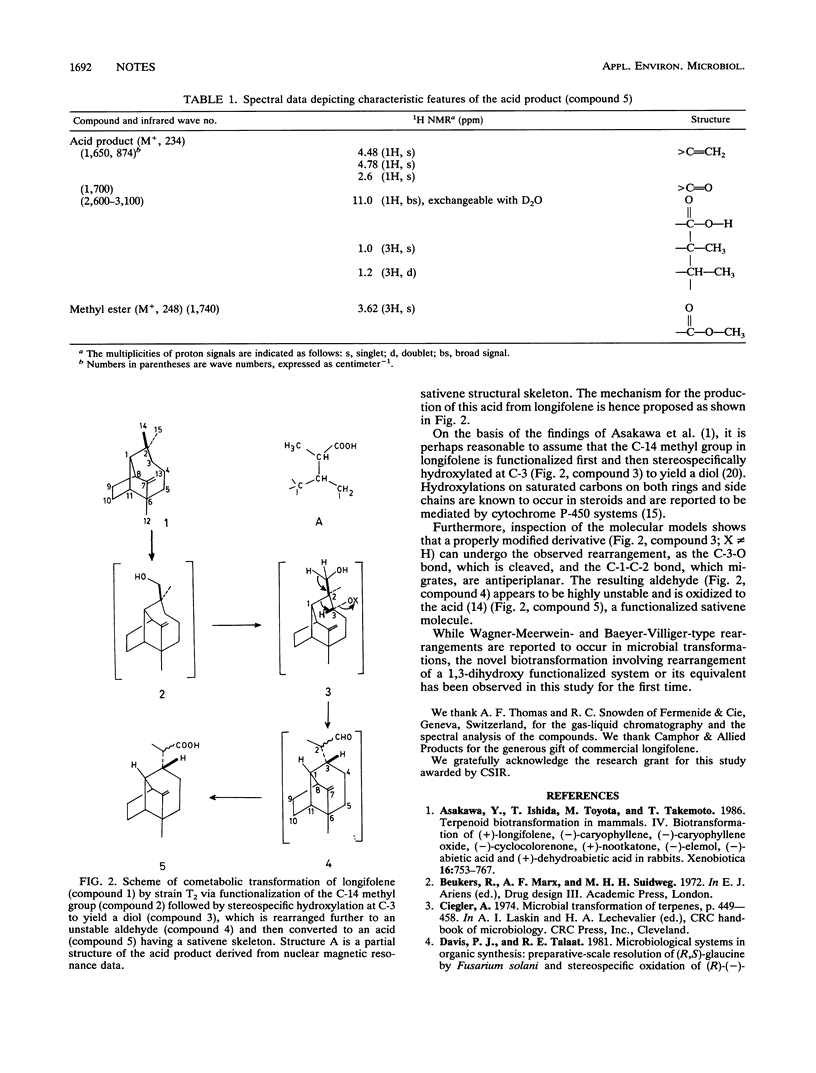
Arthrobacter ilicis T2 brings about a unique type of cometabolic structural rearrangement of longifolene, a sesquiterpene, resulting in the formation of an acid. Infrared, nuclear magnetic resonance, mass spectrometry, and decoupling studies indicate that the acid product has a sativenelike structure, which is confirmed by conversion of the acid to its methyl ester and hydrocarbon.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakawa Y., Ishida T., Toyota M., Takemoto T. Terpenoid biotransformation in mammals. IV Biotransformation of (+)-longifolene, (-)-caryophyllene, (-)-caryophyllene oxide, (-)-cyclocolorenone, (+)-nootkatone, (-)-elemol, (-)-abietic acid and (+)-dehydroabietic acid in rabbits. Xenobiotica. 1986 Aug;16(8):753–767. doi: 10.3109/00498258609043566. [DOI] [PubMed] [Google Scholar]
- DE MAYO P., WILLIAMS R. E. SATIVENE, PARENT OF THE TOXIN FROM HELMINTHOSPORIUM SATIVUM. J Am Chem Soc. 1965 Jul 20;87:3275–3275. doi: 10.1021/ja01092a066. [DOI] [PubMed] [Google Scholar]
- Davis P. J., Talaat R. E. Microbiological Systems in Organic Synthesis: Preparative-Scale Resolution of (RS)-Glaucine by Fusarium solani and Stereospecific Oxidation of (R)-(-)-Glaucine by Aspergillus flavipes. Appl Environ Microbiol. 1981 May;41(5):1243–1247. doi: 10.1128/aem.41.5.1243-1247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R. S. Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriol Rev. 1972 Jun;36(2):146–155. doi: 10.1128/br.36.2.146-155.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan D. C., Fu P. P., Cerniglia C. E. Stereoselective fungal metabolism of 7,12-dimethylbenz[a]anthracene: identification and enantiomeric resolution of a K-region dihydrodiol. Appl Environ Microbiol. 1987 Oct;53(10):2560–2566. doi: 10.1128/aem.53.10.2560-2566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J. J. Microbial cooxidations involving hydrocarbons. Microbiol Rev. 1979 Mar;43(1):59–72. doi: 10.1128/mr.43.1.59-72.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson K. E., Fu P. P., Cerniglia C. E. Metabolism of 1-, 3-, and 6-nitrobenzo[a]pyrene by intestinal microflora. J Toxicol Environ Health. 1988;23(4):527–537. doi: 10.1080/15287398809531134. [DOI] [PubMed] [Google Scholar]
- Rosazza J. P., Smith R. V. Microbial models for drug metabolism. Adv Appl Microbiol. 1979;25:169–208. doi: 10.1016/s0065-2164(08)70150-3. [DOI] [PubMed] [Google Scholar]


