Abstract
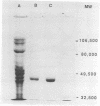
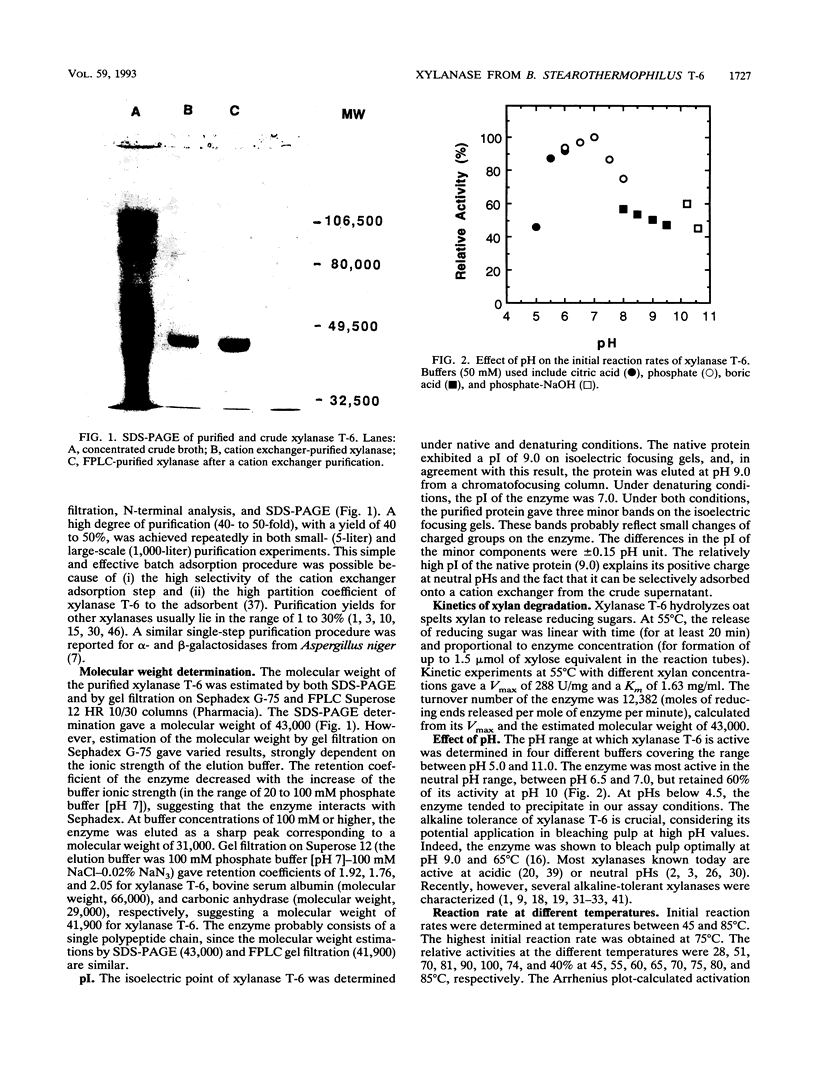
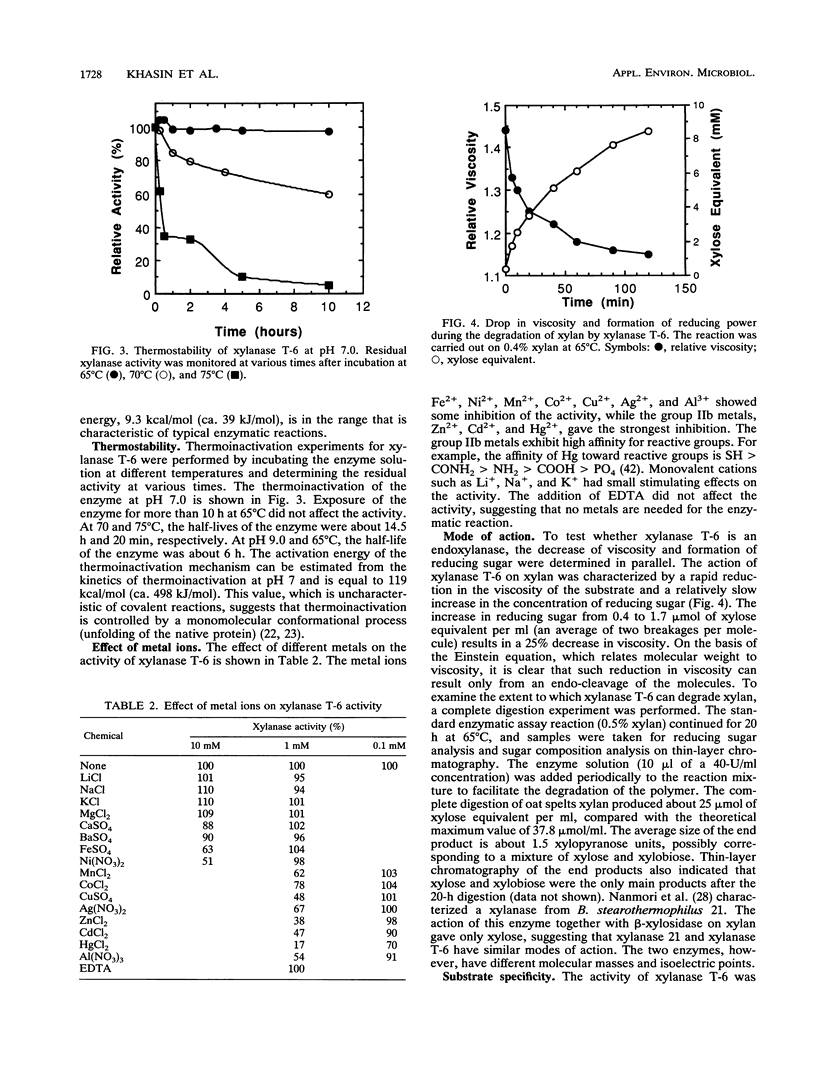
Bacillus stearothermophilus T-6 produces an extracellular xylanase that was shown to optimally bleach pulp at pH 9 and 65 degrees C. The enzyme was purified and concentrated in a single adsorption step onto a cation exchanger and is made of a single polypeptide with an apparent M(r) of 43,000 (determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis). Xylanase T-6 is an endoxylanase that completely degrades xylan to xylose and xylobiose. The pIs of the purified protein were 9 and 7 under native and denaturing conditions, respectively. The optimum activity was at pH 6.5; however, 60% of the activity was still retained at pH 10. At 65 degrees C and pH 7, the enzyme was stable for more than 10 h; at 65 degrees C and pH 9, the half-life of the enzyme was approximately 6 h. Kinetic experiments at 55 degrees C gave Vmax and Km values of 288 U/mg and 1.63 mg/ml, respectively. The enzyme had no apparent requirement for cofactors, and its activity was strongly inhibited by Zn2+, Cd2+, and Hg2+. Xylan completely protected the protein from inactivation by N-bromosuccinimide. The N-terminal sequence of the first 45 amino acids of the enzyme showed high homology with the N-terminal region of xylanase A from the alkalophilic Bacillus sp. strain C-125.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernier R., Desrochers M., Jurasek L., Paice M. G. Isolation and Characterization of a Xylanase from Bacillus subtilis. Appl Environ Microbiol. 1983 Aug;46(2):511–514. doi: 10.1128/aem.46.2.511-514.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Analytical isoelectric focusing using a high-voltage vertical slab polyacrylamide gel system. Anal Biochem. 1984 Nov 1;142(2):421–436. doi: 10.1016/0003-2697(84)90486-x. [DOI] [PubMed] [Google Scholar]
- Honda H., Kudo T., Horikoshi K. Molecular cloning and expression of the xylanase gene of alkalophilic Bacillus sp. strain C-125 in Escherichia coli. J Bacteriol. 1985 Feb;161(2):784–785. doi: 10.1128/jb.161.2.784-785.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John M., Schmidt B., Schmidt J. Purification and some properties of five endo-1,4-beta-D-xylanases and a beta-D-xylosidase produced by a strain of Aspergillus niger. Can J Biochem. 1979 Feb;57(2):125–134. doi: 10.1139/o79-016. [DOI] [PubMed] [Google Scholar]
- Keskar S. S., Srinivasan M. C., Deshpande V. V. Chemical modification of a xylanase from a thermotolerant Streptomyces. Evidence for essential tryptophan and cysteine residues at the active site. Biochem J. 1989 Jul 1;261(1):49–55. doi: 10.1042/bj2610049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klibanov A. M. Stabilization of enzymes against thermal inactivation. Adv Appl Microbiol. 1983;29:1–28. doi: 10.1016/s0065-2164(08)70352-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nanmori T., Watanabe T., Shinke R., Kohno A., Kawamura Y. Purification and properties of thermostable xylanase and beta-xylosidase produced by a newly isolated Bacillus stearothermophilus strain. J Bacteriol. 1990 Dec;172(12):6669–6672. doi: 10.1128/jb.172.12.6669-6672.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Reilly P. J. Xylanases: structure and function. Basic Life Sci. 1981;18:111–129. doi: 10.1007/978-1-4684-3980-9_8. [DOI] [PubMed] [Google Scholar]
- Wong K. K., Tan L. U., Saddler J. N. Multiplicity of beta-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev. 1988 Sep;52(3):305–317. doi: 10.1128/mr.52.3.305-317.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]