Abstract
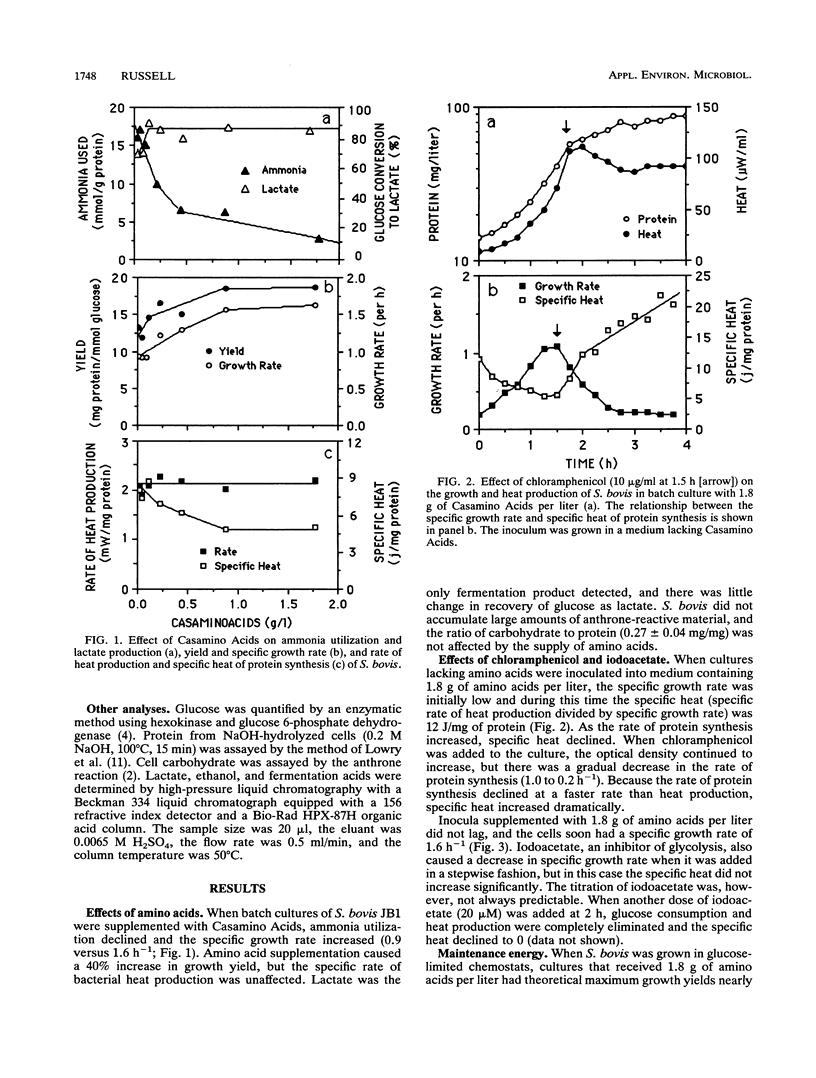
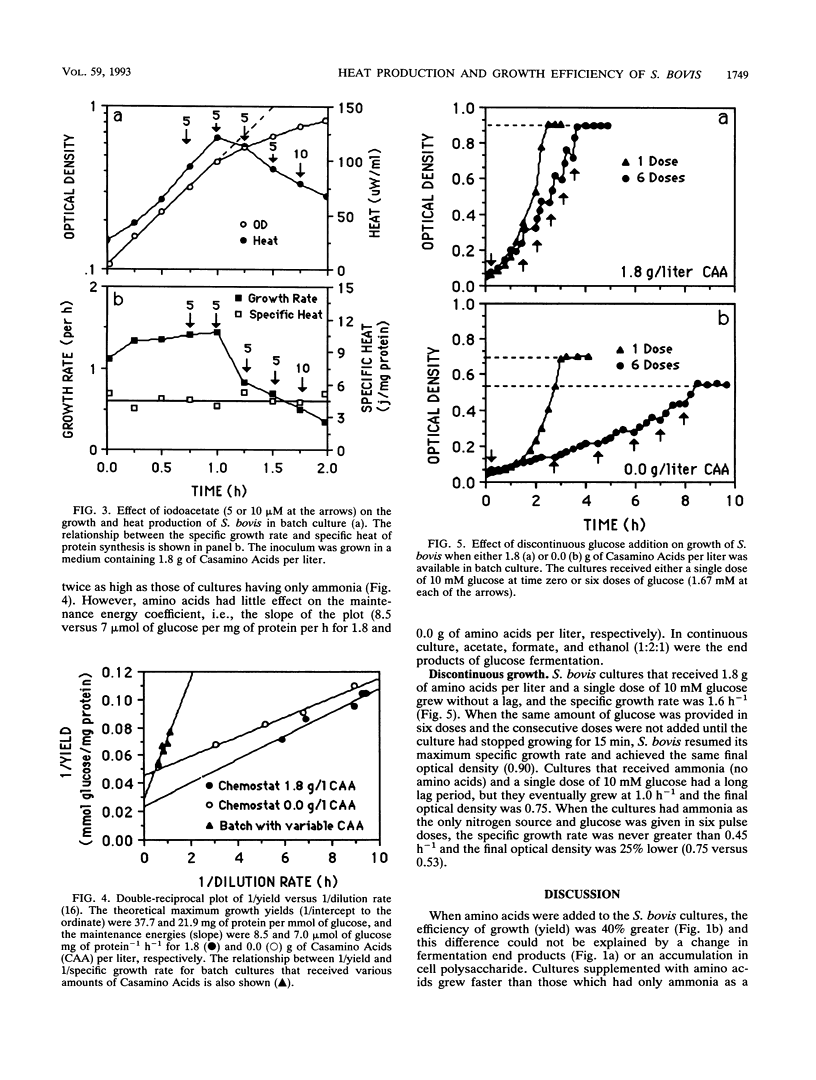
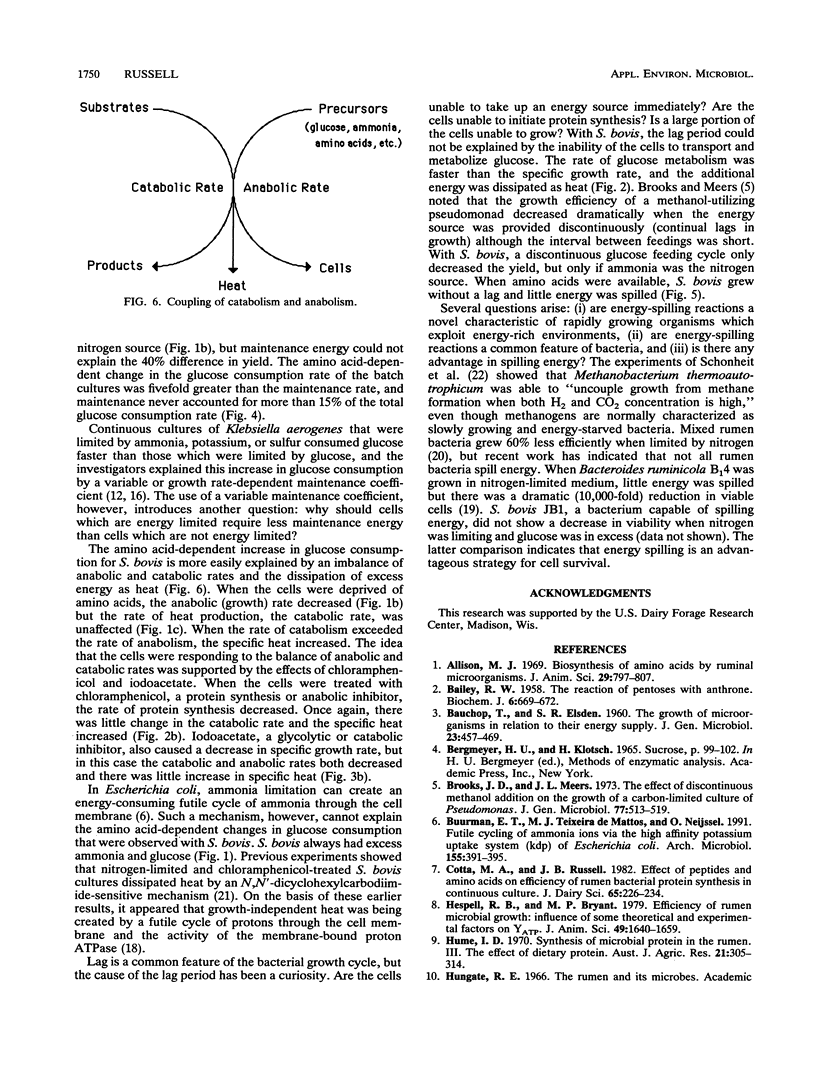
Streptococcus bovis JB1 grew nearly twice as fast (0.9 versus 1.6 h-1) and had a 40% greater growth yield (18 versus 12.5 mg of protein per mmol of glucose) when an ammonia-based medium was supplemented with amino acids, but the glucose consumption rate (88 mumol mg of protein-1 h-1) and specific rate of heat production (2.1 mW/mg of protein) were unaffected. Amino acid availability had little effect on the catabolic rate, but the specific heat decreased 40% (8.8 to 5.2 J/mg of protein). These growth rate-dependent changes in metabolic efficiency were fivefold greater than the maintenance energy. Chloramphenicol (100 mg/l), an inhibitor of protein synthesis, caused a gradual decrease in anabolic (growth) rate, but there was little change in the rate of glucose consumption and the specific heat increased. When growth was inhibited by iodoacetate, the catabolic and anabolic rates both declined and there was not increase in specific heat. On the basis of these results, the benefit of amino acid supplementation was largely explained by the balance of anabolic and catabolic rates. When amino acids were available, the anabolic and catabolic rates were more closely matched and less energy was spilled as heat.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. J. Biosynthesis of amono acids by ruminal microorganisms. J Anim Sci. 1969 Nov;29(5):797–807. doi: 10.2527/jas1969.295797x. [DOI] [PubMed] [Google Scholar]
- BAILEY R. W. The reaction of pentoses with anthrone. Biochem J. 1958 Apr;68(4):669–672. doi: 10.1042/bj0680669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- Buurman E. T., Teixeira de Mattos M. J., Neijssel O. M. Futile cycling of ammonium ions via the high affinity potassium uptake system (Kdp) of Escherichia coli. Arch Microbiol. 1991;155(4):391–395. doi: 10.1007/BF00243460. [DOI] [PubMed] [Google Scholar]
- Hespell R. B., Bryant M. P. Efficiency of rumen microbial growth: influence of some theoretical and experimental factors of YATP. J Anim Sci. 1979 Dec;49(6):1640–1659. doi: 10.2527/jas1979.4961640x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neijssel O. M., Tempest D. W. Bioenergetic aspects of aerobic growth of Klebsiella aerogenes NCTC 418 in carbon-limited and carbon-sufficient chemostat culture. Arch Microbiol. 1976 Mar 19;107(2):215–221. doi: 10.1007/BF00446843. [DOI] [PubMed] [Google Scholar]
- Neijssel O. M., Tempest D. W. The role of energy-spilling reactions in the growth of Klebsiella aerogenes NCTC 418 in aerobic chemostat culture. Arch Microbiol. 1976 Nov 2;110(23):305–311. doi: 10.1007/BF00690243. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- Russell J. B. A re-assessment of bacterial growth efficiency: the heat production and membrane potential of Streptococcus bovis in batch and continuous culture. Arch Microbiol. 1991;155(6):559–565. doi: 10.1007/BF00245350. [DOI] [PubMed] [Google Scholar]
- Russell J. B. Glucose toxicity and inability of Bacteroides ruminicola to regulate glucose transport and utilization. Appl Environ Microbiol. 1992 Jun;58(6):2040–2045. doi: 10.1128/aem.58.6.2040-2045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B. Heat production by ruminal bacteria in continuous culture and its relationship to maintenance energy. J Bacteriol. 1986 Nov;168(2):694–701. doi: 10.1128/jb.168.2.694-701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J. ATPase-dependent energy spilling by the ruminal bacterium, Streptococcus bovis. Arch Microbiol. 1990;153(4):378–383. doi: 10.1007/BF00249009. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J. Concentration of ammonia across cell membranes of mixed rumen bacteria. J Dairy Sci. 1987 May;70(5):970–976. doi: 10.3168/jds.S0022-0302(87)80101-7. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H. A theoretical study on the amount of ATP required for synthesis of microbial cell material. Antonie Van Leeuwenhoek. 1973;39(3):545–565. doi: 10.1007/BF02578899. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Neijssel O. M. The status of YATP and maintenance energy as biologically interpretable phenomena. Annu Rev Microbiol. 1984;38:459–486. doi: 10.1146/annurev.mi.38.100184.002331. [DOI] [PubMed] [Google Scholar]
- WOLIN M. J., MANNING G. B., NELSON W. O. Ammonium salts as a sole source of nitrogen for the growth of Streptococcus bovis. J Bacteriol. 1959 Jul;78(1):147–147. doi: 10.1128/jb.78.1.147-147.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]



