Abstract
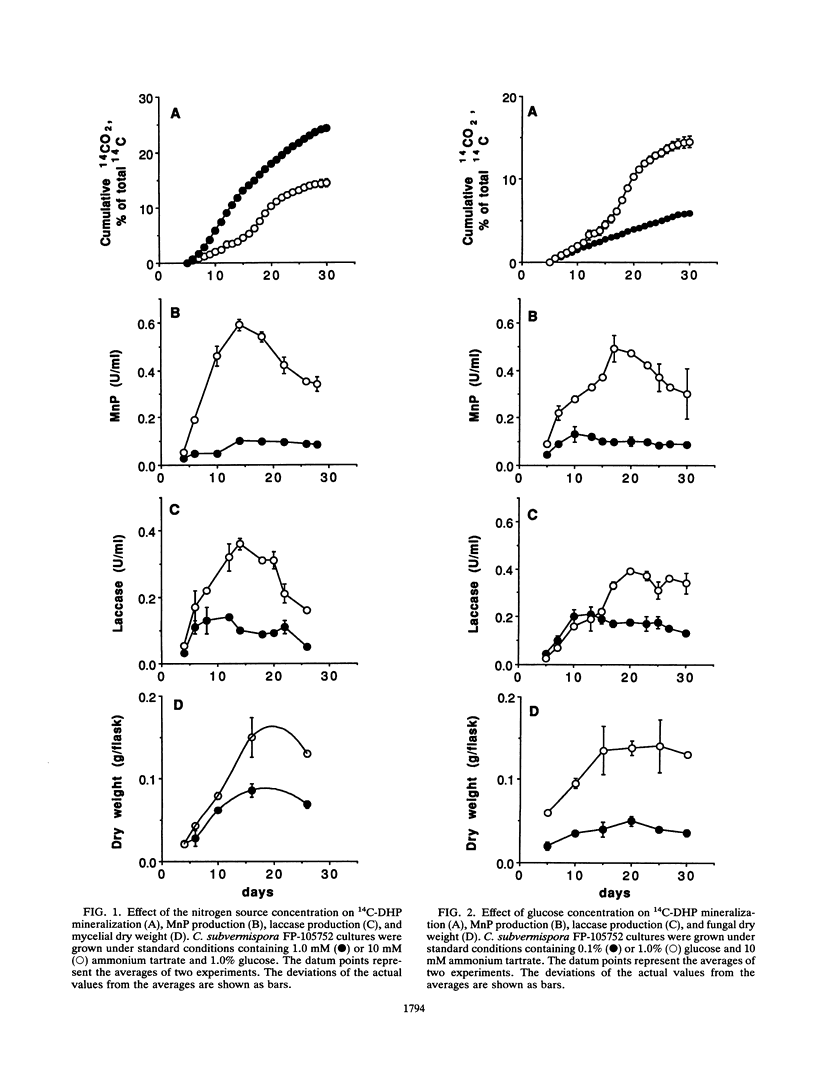
The ability of the white rot fungus Ceriporiopsis subvermispora to mineralize 14C-synthetic lignin was studied under different culture conditions, and the levels of two extracellular enzymes were monitored. The highest mineralization rates (28% after 28 days) were obtained in cultures containing a growth-limiting amount of nitrogen source (1.0 mM ammonium tartrate); under this condition, the levels of manganese peroxidase (MnP) and laccase present in the culture supernatant solutions were very low compared with cultures containing 10 mM of the nitrogen source. In contrast, cultures containing a limiting concentration of the carbon source (0.1% glucose) showed low levels of both enzymes and also very low mineralization rates compared with cultures containing 1% glucose. Cultures containing 11 ppm of Mn(II) showed a higher rate of mineralization than those containing 0.3 or 40 ppm of this cation. Levels of MnP and laccase were higher when 40 ppm of Mn(II) was used. Mineralization rates were slightly higher in cultures flushed daily with oxygen, whereas laccase levels were lower and MnP levels were approximately the same as in cultures maintained under an air atmosphere. The presence of 0.4 mM veratryl alcohol reduced both mineralization rates and MnP levels, without affecting laccase levels. Lignin peroxidase activity was not detected under any condition. Addition of purified lignin peroxidase to the cultures in the presence or absence of veratryl alcohol did not enhance mineralization rates significantly.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banci L., Bertini I., Turano P., Tien M., Kirk T. K. Proton NMR investigation into the basis for the relatively high redox potential of lignin peroxidase. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6956–6960. doi: 10.1073/pnas.88.16.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnarme P., Jeffries T. W. Mn(II) Regulation of Lignin Peroxidases and Manganese-Dependent Peroxidases from Lignin-Degrading White Rot Fungi. Appl Environ Microbiol. 1990 Jan;56(1):210–217. doi: 10.1128/aem.56.1.210-217.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boominathan K., Dass S. B., Randall T. A., Kelley R. L., Reddy C. A. Lignin peroxidase-negative mutant of the white-rot basidiomycete Phanerochaete chrysosporium. J Bacteriol. 1990 Jan;172(1):260–265. doi: 10.1128/jb.172.1.260-265.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbonnais R., Paice M. G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990 Jul 2;267(1):99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- Brown J. A., Alic M., Gold M. H. Manganese peroxidase gene transcription in Phanerochaete chrysosporium: activation by manganese. J Bacteriol. 1991 Jul;173(13):4101–4106. doi: 10.1128/jb.173.13.4101-4106.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A., Bettermann A., Kirk T. K. Identification of a specific manganese peroxidase among ligninolytic enzymes secreted by Phanerochaete chrysosporium during wood decay. Appl Environ Microbiol. 1991 May;57(5):1453–1460. doi: 10.1128/aem.57.5.1453-1460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Factors Involved in the Regulation of a Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Feb;49(2):299–304. doi: 10.1128/aem.49.2.299-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester I. T., Grabski A. C., Mishra C., Kelley B. D., Strickland W. N., Leatham G. F., Burgess R. R. Characteristics and N-terminal amino acid sequence of a manganese peroxidase purified from Lentinula edodes cultures grown on a commercial wood substrate. Appl Microbiol Biotechnol. 1990 Jun;33(3):359–365. doi: 10.1007/BF00164536. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Gold M. H. Purification and characterization of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys. 1985 Nov 1;242(2):329–341. doi: 10.1016/0003-9861(85)90217-6. [DOI] [PubMed] [Google Scholar]
- Glenn J. K., Morgan M. A., Mayfield M. B., Kuwahara M., Gold M. H. An extracellular H2O2-requiring enzyme preparation involved in lignin biodegradation by the white rot basidiomycete Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1077–1083. doi: 10.1016/0006-291x(83)90672-1. [DOI] [PubMed] [Google Scholar]
- Jeffries T. W., Choi S., Kirk T. K. Nutritional Regulation of Lignin Degradation by Phanerochaete chrysosporium. Appl Environ Microbiol. 1981 Aug;42(2):290–296. doi: 10.1128/aem.42.2.290-296.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser P., Kirk T. K., Zeikus J. G. Ligninolytic enzyme system of Phanaerochaete chrysosporium: synthesized in the absence of lignin in response to nitrogen starvation. J Bacteriol. 1978 Sep;135(3):790–797. doi: 10.1128/jb.135.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk T. K., Connors W. J., Bleam R. D., Hackett W. F., Zeikus J. G. Preparation and microbial decomposition of synthetic [14C]ligins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2515–2519. doi: 10.1073/pnas.72.7.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth A. B., Denny M., Tien M. Overproduction of lignin-degrading enzymes by an isolate of Phanerochaete chrysosporium. Appl Environ Microbiol. 1991 Sep;57(9):2591–2596. doi: 10.1128/aem.57.9.2591-2596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez J., Jeffries T. W. Mineralization of C-Ring-Labeled Synthetic Lignin Correlates with the Production of Lignin Peroxidase, not of Manganese Peroxidase or Laccase. Appl Environ Microbiol. 1990 Jun;56(6):1806–1812. doi: 10.1128/aem.56.6.1806-1812.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périé F. H., Gold M. H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl Environ Microbiol. 1991 Aug;57(8):2240–2245. doi: 10.1128/aem.57.8.2240-2245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-degrading enzyme from Phanerochaete chrysosporium: Purification, characterization, and catalytic properties of a unique H(2)O(2)-requiring oxygenase. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2280–2284. doi: 10.1073/pnas.81.8.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien M., Myer S. B. Selection and characterization of mutants of Phanerochaete chrysosporium exhibiting ligninolytic activity under nutrient-rich conditions. Appl Environ Microbiol. 1990 Aug;56(8):2540–2544. doi: 10.1128/aem.56.8.2540-2544.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991 Apr 15;176(1):269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]



