Abstract
We have identified an opsin, melanopsin, in photosensitive dermal melanophores of Xenopus laevis. Its deduced amino acid sequence shares greatest homology with cephalopod opsins. The predicted secondary structure of melanopsin indicates the presence of a long cytoplasmic tail with multiple putative phosphorylation sites, suggesting that this opsin’s function may be finely regulated. Melanopsin mRNA is expressed in hypothalamic sites thought to contain deep brain photoreceptors and in the iris, a structure known to be directly photosensitive in amphibians. Melanopsin message is also localized in retinal cells residing in the outermost lamina of the inner nuclear layer where horizontal cells are typically found. Its expression in retinal and nonretinal tissues suggests a role in vision and nonvisual photoreceptive tasks, such as photic control of skin pigmentation, pupillary aperture, and circadian and photoperiodic physiology.
Photopigments consist of an integral membrane apoprotein, opsin, that is covalently linked to a retinaldehyde chromophore. Upon illumination, the chromophore is photoisomerized, forcing a conformational change in the surrounding opsin and subsequently initiating an intracellular signaling cascade. The conversion of light into neural signals within photoreceptor cells of the retina is the best studied example of opsin-mediated signaling.
Dermal melanophores of Xenopus laevis, like retinal photoreceptors, are photosensitive (1). When maintained in vitro, the melanosomes in dermal melanophores migrate to the cellular periphery in response to illumination (2) or the activation of a wide variety of G protein-coupled receptors (3). The melanophore response to these agents has led to the development of innovative systems for studying functional interactions between ligands and their receptors (4, 5). Melanophore photosensitivity is reversibly activated by retinaldehydes (6) and has an action spectrum characteristic of opsin-like photopigments (2). In an effort to identify an opsin that may regulate melanosome migration, we have analyzed protein extracts of cultured melanophores for opsin immunoreactivity and screened a melanophore cDNA library for opsin-like nucleotide sequences.
MATERIALS AND METHODS
Western Blot.
Approximately 105 cultured Xenopus laevis dermal melanophores were washed twice with 1× calcium- and magnesium-free Dulbecco’s phosphate buffer and lysed with lysis buffer [1% IGEPAL CA-630 (Sigma)/0.2 M NaBH3CN/1 mM phenylmethylsulfonyl fluoride/aprotinin (0.1 trypsin inhibitor unit/ml)/5 mM EDTA/0.086 M NaC2H3O2, pH 5.0, 4°C]. A homogenate of one early postmetamorphic adult eye was also extracted as above. Lysates were centrifuged and the supernatants subjected to SDS/PAGE analysis and subsequent electroblotting onto a poly(vinylidene difluoride) membrane. The blot was probed with a 1:2,000 dilution of antisera (CERN 886) raised against bovine rhodopsin and detected by enhanced chemiluminescence.
cDNA Library Screen.
A X. laevis dermal melanophore oligo(dT) cDNA library was screened with a mixture of 32P-labeled probes. Probes were synthesized by random priming of TA-cloned (Invitrogen) fragments of X. laevis rhodopsin (7) (759 bp) and violet opsin (8) (279 bp) cDNAs. Phage DNA was isolated from positive clones and sequenced in both directions by using λgt10 universal primers. Internal sequencing primers were designed from newly acquired sequence data until both strands of the insert were completely sequenced. The insert sequence was confirmed by PCR amplification and sequencing of the entire melanopsin ORF from fresh melanophore cDNA. All sequences were assembled by using the seqman program within the DNAstar software suite (Lasergene, Madison, WI). Sequences were compared with the GenBank database by using the blast algorithm (9).
In Situ Hybridization Histochemistry.
Methods are detailed elsewhere (10). Briefly, adult and tadpole (stages 56 and 57) serial sections were incubated for 16–20 h with sense or antisense 35S-labeled RNA probes representing bases 957–1,553 of the melanopsin ORF, which correspond to the unique cytoplasmic tail. After incubation, unhybridized transcripts were digested with RNase A and sections were washed at high stringency in 0.1× SSC at 65°C. Emulsion-dipped sections were then exposed for 6 weeks. Frog staging (11) and neuroanatomy (12) were according to established criteria.
RESULTS
Anti-Opsin Immunoreactivity in Melanophores.
Photosensitive melanophores contain a protein that is immunoreactive to an antiserum raised against bovine rhodopsin (Fig. 1). The immunoreactive band has a molecular mass approximately 50% greater than the major 35-kDa rhodopsin band from whole Xenopus eye extract. The discrepancy between the predicted molecular mass of rhodopsin (40 kDa) and that observed after SDS/PAGE is a phenomenon commonly found among opsins and other integral membrane proteins (13). A minor protein band from whole Xenopus eye extract had the same molecular weight as that observed from the melanophore extract.
Figure 1.
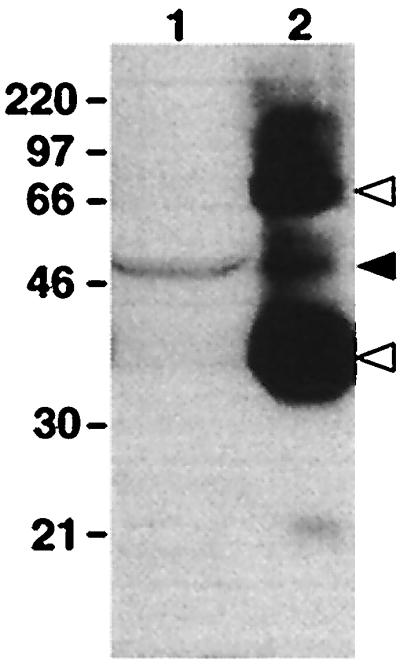
Western blot of protein extracts from dermal melanophores and whole eye probed with an antiserum raised against bovine rhodopsin. Indicated molecular masses are in kDa. Lanes: 1, total protein from cultured melanophores; 2, 1% of total protein from a whole early postmetamorphic adult eye. A 50-kDa immunoreactive band is present in both lanes (solid arrowhead). The 35- and 70-kDa bands in lane 2 are monomeric and dimeric rhodopsin, respectively (open arrowheads).
Identification of an Opsin.
A 2.2-kb cDNA clone was isolated by screening a X. laevis dermal melanophore cDNA library. This clone contains a 1.6-kb ORF encoding a 534-amino acid protein that is more homologous to known opsins than other families of G protein-coupled receptors. We have named this opsin melanopsin because of its isolation from a melanophore cDNA library.
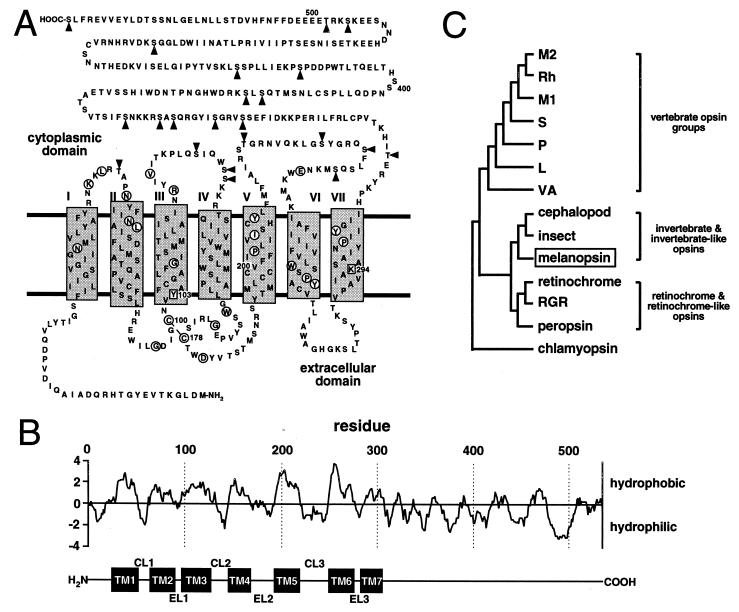
Melanopsin shares structural similarities with all known opsins including an extracellular amino terminus and seven transmembrane domains, as determined by tmpred software (14) (Fig. 2 A and B). Other similarities include a lysine (Lys-294) in the seventh transmembrane domain to serve as a site for Schiff’s base linkage of the chromophore (16) and a pair of cysteines (Cys-100 and Cys-178) in the second and third extracellular loops to stabilize the tertiary structure by disulfide bridge formation (17). There is a notable absence of N-glycosylation sites typically found in the extracellular amino terminus of most opsins.
Figure 2.
Melanopsin structure and phylogeny. (A) Deduced amino acid sequence and predicted secondary structure of melanopsin. Transmembrane domains are designated by shaded rectangles and circled residues are identical to at least 65 of 67 aligned vertebrate and invertebrate opsins (ftp://swift.embl-heidelberg.de/tm7/align/opsins.ALIGN). Lys-294 is a putative site for chromophore attachment via a Schiff’s base linkage, and Tyr-103 is the aromatic residue conserved in all invertebrates and possibly involved in stabilizing the chromophore. Arrowheads indicate potential intracellular phosphorylation sites. (B) Hydropathy analysis [Kyte–Doolittle algorithm (15); window, 10 amino acids] predicts a secondary structure consistent with the tmpred algorithm. The corresponding transmembrane domains (TM1 to TM7), cytoplasmic loops (CL1 to CL3), and extracellular loops (EL1 to EL3) are indicated below the plot. (C) A phylogenetic tree was constructed by aligning melanopsin against representatives of the vertebrate opsin groups (L, long-wavelength-sensitive opsin; M1, blue-like middle-wavelength-sensitive opsin; M2, green-like middle-wavelength-sensitive opsin; P, pineal opsin; Rh, rhodopsin; S, short-wavelength-sensitive—all from chicken; VA, vertebrate ancient opsin—from Atlantic salmon), representatives of the retinochrome-like opsins (retinochrome from squid and RGR and peropsin from human), and representatives from cephalopods (octopus rhodopsin), insects (Drosophila Rh1 opsin), and unicellular eukaryotes (Chlamydomonas chlamyopsin). All sequences are available through GenBank.
Despite melanopsin’s vertebrate origin, it is more homologous to invertebrate opsins. It has a deduced amino acid sequence that shares 39% identity with octopus rhodopsin and approximately 30% identity with typical vertebrate opsins (Table 1). This homology to the invertebrate opsins is reflected in several domains thought to have functional significance (Fig. 3). In particular, melanopsin has an aromatic residue (Tyr-103) in the third transmembrane helix that may be a component of a larger complex involved in stabilizing the Schiff’s base (18). The corresponding vertebrate residue is acidic (19, 20). Also, like invertebrate opsins, melanopsin has an insertion (Asn-225 to Gly-233) in the third cytoplasmic loop, albeit the similarity to invertebrate opsins at the amino acid level is limited. Such an insertion is absent in the vertebrate opsins. This third cytoplasmic loop determines the family of G protein that is activated by heptahelical receptors (21, 22). Melanopsin’s conspicuously long cytoplasmic tail contains 14 potential phosphorylation sites, suggesting that its activation state may be regulated by kinases (23).
Table 1.
Amino acid comparison of melanopsin to the other opsins
| Opsin/family* | % identity |
|---|---|
| M2 | 31 |
| Rh | 30 |
| M1 | 29 |
| S | 30 |
| P | 30 |
| L | 31 |
| VA | 30 |
| Cephalopod | 39 |
| Insect | 32 |
| Retinochrome | 25 |
| RGR | 30 |
| Peropsin | 32 |
| Chlamyopsin | 18 |
Data are from pairwise alignments of amino acid sequences contained within the predicted first through seventh transmembrane domains.
Representatives of opsin families are described in Fig. 2C.
Figure 3.
Alignment of melanopsin amino acid sequence with those of octopus rhodopsin, Xenopus red opsin, Xenopus violet opsin, and Xenopus rhodopsin. Predicted transmembrane domains are overlined. Conserved sites of chromophore linkage (★) and disulfide bridge formation (•) are indicated. The residues corresponding to the acidic counterion in the vertebrate visual pigments are also indicated (▴). The peptide insertion within melanopsin’s third cytoplasmic loop has been shaded.
Phylogenetic analysis indicates that melanopsin and the invertebrate opsins diverged from a common ancestor (Fig. 2C). Bootstrapping demonstrates that this node is well resolved (485 of 500 trees). The alignment and distance matrix was generated with clustal w 1.6 (24), and the subsequent phylogenetic tree was constructed by using the neighbor joining method (25). Placement of the vertebrate opsin groups agrees with published trees (26, 27).
Localization of Melanopsin mRNA by in Situ Hybridization.
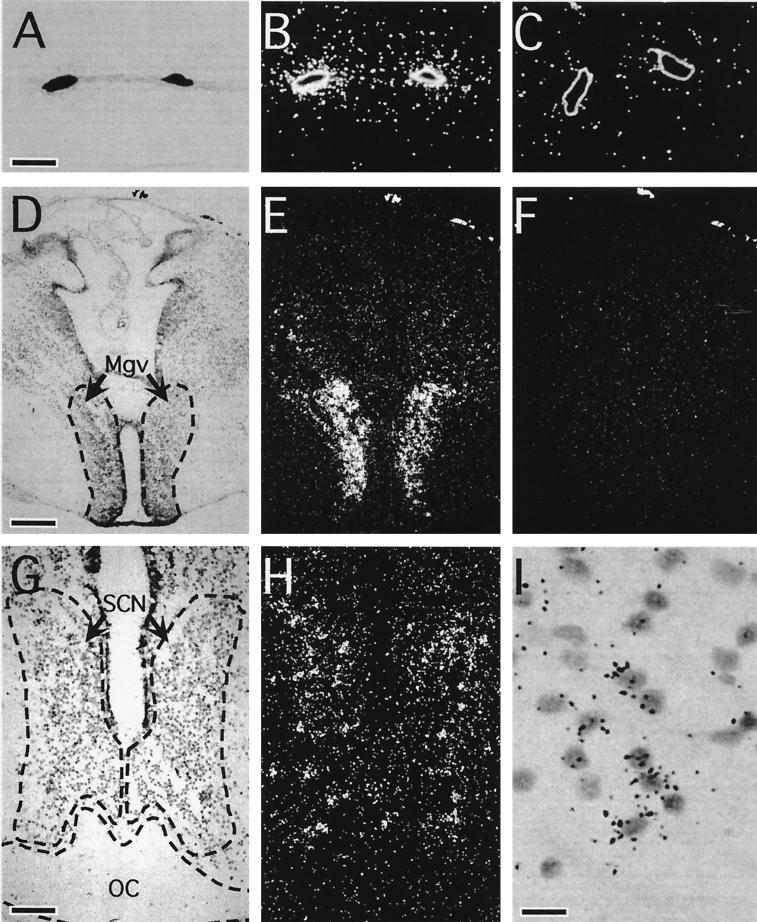
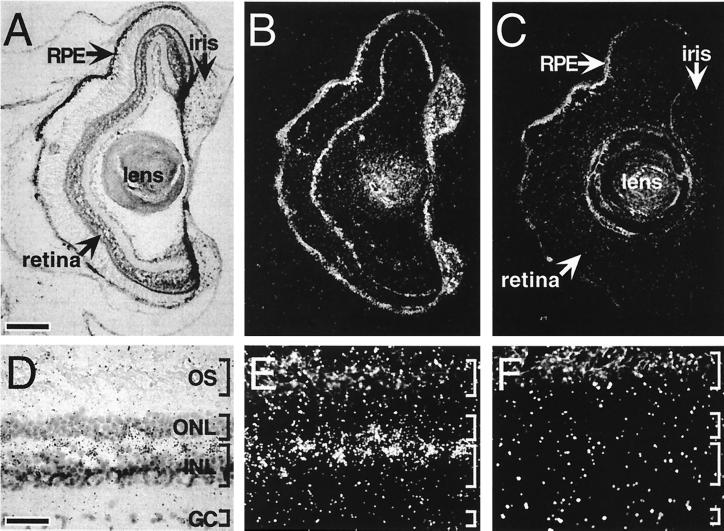
Because of its initial isolation from a melanophore cDNA library, the presence of melanopsin message in dermal melanophores was predicted (Fig. 4 A–C). Unexpectedly, melanopsin message is also localized to several structures within the brain. Transcripts are present in the ventral part of the magnocellular preoptic nucleus (Fig. 4 D–F) and the suprachiasmatic nucleus (SCN; Fig. 4 G–I). In addition, we have identified melanopsin message in at least three ocular sites: iris, retinal pigment epithelium (RPE), and inner retina (Fig. 5). Melanopsin is most highly expressed within the iris; a relatively low level of mRNA expression is observed in the RPE. Perhaps most interesting is the high level of expression within nonphotoreceptor cells of the retina. These retinal cells are located in the outermost lamina of the inner nuclear layer where horizontal cells are typically found.
Figure 4.
Bright-field and dark-field photomicrographs of melanopsin transcript distribution within extraocular structures. Melanopsin message was localized to tadpole (stages 56 and 57) dermal melanophores (A and B), the ventral aspect of the magnocellular preoptic nucleus (Mgv) (D and E), and the SCN (G and H) of the tadpole brain (stages 56 and 57). Cell-specific antisense hybridization was restricted to a subset of SCN cells (I). In contrast, incubation with the sense control probe yielded no hybridization above background (C and F). (Corresponding bright-field views are not shown.) OC, optic chiasm. [Bars = 20 μm (A), 150 μm (D), 75 μm (G), and 15 μm (I).]
Figure 5.
Bright-field and dark-field photomicrographs of melanopsin transcript distribution within ocular structures. In sections of tadpole (stages 56 and 57) (A and B) and adult (D and E) eye, melanopsin transcripts were identified in retinal cells within the outer lamina of the inner nuclear layer (INL) (A, B, D, and E). In contrast, incubation of adjacent sections with the sense RNA probe (C and F) resulted in no labeling above background. (Corresponding bright-field views are not shown.) Intense cell-specific hybridization was also observed in the iris and less intensely within the RPE. GC, ganglion cell layer; ONL, outer nuclear layer; OS, photoreceptor outer segments. [Bars = 150 μm (A) and 35 μm (D).]
DISCUSSION
The retinaldehyde dependence (6) and action spectrum (2) for light-induced melanosome migration strongly implicates an opsin in melanophore photosensitivity. The molecular mass of the anti-rhodopsin immunoreactive band observed on Western blots of melanophore protein extracts suggested that this opsin significantly differs from typical retinal opsins. The subsequent identification and in situ localization of melanopsin, a relatively massive and unique opsin, is consistent with these findings.
The existence of deep brain photoreceptors has been known for almost a century (28), but their exact identity has been difficult to elucidate. Deep brain photoreceptors are known to regulate circadian rhythms and photoperiodic responses in many nonmammalian vertebrate species. These responses have been best characterized in birds (29) and reptiles (30) but are also found in amphibians (31). Immunohistochemical studies with anti-opsin antibodies have identified immunoreactive cells in the brain (32, 33), although the identities of the antigens have been elusive. Anti-bovine rhodopsin immunoreactivity has been observed within the SCN (34) and magnocellular preoptic nucleus of the frog (34, 35). The SCN is a primary circadian pacemaker in many vertebrates, although its function in frogs has not been characterized. Melanopsin may provide a mechanism by which light directly affects pacemaker cells within the SCN, thus regulating the expression of circadian rhythms.
The isolated amphibian iris is directly light sensitive (36). An opsin-based photopigment has been suspected of mediating this sensitivity because the action spectrum for the pupillary constriction response is similar to the absorption spectrum of frog rhodopsin (37). Localization of melanopsin to the iris suggests that it may be subserving iridial photomechanical movement.
In the vertebrates, photoisomerization of the 11-cis chromophore into the all-trans configuration results in its release from the meta state of the opsin. It is then actively transported to the RPE, reisomerized into the 11-cis isomer, and carried back to the photoreceptors to regenerate the bleached visual pigments. Within the last 5 years, RGR (38) and peropsin (39), like melanopsin, have been identified in the RPE. The similarity of RGR and peropsin to squid retinochrome suggests that they may function as photoisomerases. Melanopsin’s peptide sequence, however, more closely resembles that of a phototransducing opsin than that of a retinal-binding protein or retinal photoisomerase. For example, residues Val-128, Gly-173, Ile-208, and the Lys/Arg-Gly/Pro dipeptide in the second cytoplasmic loop are conserved in melanopsin and in more than 96% of other known opsins but are absent in RGR, peropsin, and retinochrome.
Invertebrate photopigments have a thermally stable metastate and the covalently linked chromophore is retained (18). Such chromophore stability allows repeated photochemical interconversion between the two states, thus eliminating the need for a closely associated RPE-like tissue to constantly supply 11-cis chromophore. Melanopsin’s invertebrate-like character suggests that it may not require an adjacent RPE-like tissue to be functional. This independence would permit melanopsin to be functional in broadly distributed anatomical sites.
The discovery of retinaldehyde-based photopigments in bacteria (40), algae (41), and possibly fungi (42) underscores their evolutionary importance and their value in simply detecting the presence or absence of light, i.e., nonvisual photoreception. Melanopsin’s anatomical distribution in melanophores, the iris, and the deep brain suggests that it is used by nonvisual photoreceptors. In melanophores and the iris, it may mediate photo-induced melanosome migration and photic regulation of pupillary constriction, respectively. Its role in the deep brain and neural retina is not clear, but it might be used in photic control of circadian rhythms.
Acknowledgments
We thank Michael Flora for helpful discussions, Theodore Osgood for technical assistance, and Bobby Soni for advice on the phylogenetic analysis. This work was supported by IBN-9319344 from the National Science Foundation and RO7049 from the Uniformed Services University of the Health Sciences (both to M.D.R.).
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: RPE, retinal pigment epithelium; SCN, suprachiasmatic nucleus.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF014797).
References
- 1.Bagnara J T, Obika M. Experientia. 1967;23:155–157. doi: 10.1007/BF02135979. [DOI] [PubMed] [Google Scholar]
- 2.Daniolos A, Lerner A B, Lerner M R. Pigment Cell Res. 1990;3:38–43. doi: 10.1111/j.1600-0749.1990.tb00260.x. [DOI] [PubMed] [Google Scholar]
- 3.McClintock T S, Rising J P, Lerner M R. J Cell Physiol. 1996;167:1–7. doi: 10.1002/(SICI)1097-4652(199604)167:1<1::AID-JCP1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 4.Lerner M R. Trends Neurosci. 1994;17:142–146. doi: 10.1016/0166-2236(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 5.Quillan J M, Jayawickreme C K, Lerner M R. Proc Natl Acad Sci USA. 1995;92:2894–2898. doi: 10.1073/pnas.92.7.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rollag M D. J Exp Zool. 1996;275:20–26. [Google Scholar]
- 7.Saha M S, Grainger R M. Brain Res Mol Brain Res. 1993;17:307–318. doi: 10.1016/0169-328x(93)90016-i. [DOI] [PubMed] [Google Scholar]
- 8.Starace D M, Knox B E. J Biol Chem. 1997;272:1095–1100. doi: 10.1074/jbc.272.2.1095. [DOI] [PubMed] [Google Scholar]
- 9.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y B, Hayes W P. Dev Biol. 1994;161:48–58. doi: 10.1006/dbio.1994.1006. [DOI] [PubMed] [Google Scholar]
- 11.Nieuwkoop P D, Faber J. Normal Table of Xenopus laevis (Daudin): A Systematic and Chronological Survey of the Development from Fertilized Egg Till the End of Metamorphosis. Amsterdam: North Holland; 1956. [Google Scholar]
- 12.Neary T J, Northcutt R G. J Comp Neurol. 1983;213:262–278. doi: 10.1002/cne.902130303. [DOI] [PubMed] [Google Scholar]
- 13.Helenius A, Simons K. Biochim Biophys Acta. 1975;415:29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann K, Stoffel W. Biol Chem Hoppe-Seyler. 1993;347:166. doi: 10.1515/bchm3.1992.373.1.187. [DOI] [PubMed] [Google Scholar]
- 15.Kyte J, Doolittle R F. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 16.Wang J K, McDowell J H, Hargrave P A. Biochemistry. 1980;19:5111–5117. doi: 10.1021/bi00563a027. [DOI] [PubMed] [Google Scholar]
- 17.Karnik S S, Sakmar T P, Chen H B, Khorana H G. Proc Natl Acad Sci USA. 1988;85:8459–8463. doi: 10.1073/pnas.85.22.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gärtner W, Towner P. Photochem Photobiol. 1995;62:1–16. doi: 10.1111/j.1751-1097.1995.tb05231.x. [DOI] [PubMed] [Google Scholar]
- 19.Nathans J. Biochemistry. 1990;29:9746–9752. doi: 10.1021/bi00493a034. [DOI] [PubMed] [Google Scholar]
- 20.Zhukovsky E A, Oprian D D. Science. 1989;246:928–930. doi: 10.1126/science.2573154. [DOI] [PubMed] [Google Scholar]
- 21.Hall D, Hoon M A, Ryba N J P, Pottinger J D D, Keen J N, Saibil H R, Findlay J B C. Biochem J. 1991;274:35–40. doi: 10.1042/bj2740035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobilka B K, Kobilka T S, Daniel K, Regan J W, Caron M G, Lefkowitz R J. Science. 1988;240:1310–1316. doi: 10.1126/science.2836950. [DOI] [PubMed] [Google Scholar]
- 23.Pearson R B, Kemp B E. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- 24.Thompson J D, Higgins D G, Gibson T J. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saitou N, Nei M. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 26.Max M, McKinnon P J, Seidenman K J, Barrett R K, Applebury M L, Takahashi J S, Margolskee R F. Science. 1995;267:1502–1506. doi: 10.1126/science.7878470. [DOI] [PubMed] [Google Scholar]
- 27.Soni B G, Foster R G. FEBS Lett. 1997;406:279–283. doi: 10.1016/s0014-5793(97)00287-1. [DOI] [PubMed] [Google Scholar]
- 28.von Frisch K. Pflügers Arch Gesamte Physiol Menschen Tiere. 1911;138:319–387. [Google Scholar]
- 29.Foster R G, Follett B K, Lythgoe J N. Nature (London) 1985;313:50–52. doi: 10.1038/313050a0. [DOI] [PubMed] [Google Scholar]
- 30.Underwood H, Menaker M. J Comp Physiol. 1976;83:187–222. [Google Scholar]
- 31.Fraile B, Paniagua R, Rodriguez M C, Saez F J. J Morph. 1989;200:151–162. doi: 10.1002/jmor.1052000205. [DOI] [PubMed] [Google Scholar]
- 32.Silver R, Witkovsky P, Horvath P, Alones V, Barnstable C J, Lehman M N. Cell Tissue Res. 1988;253:189–198. doi: 10.1007/BF00221754. [DOI] [PubMed] [Google Scholar]
- 33.Foster R G, Garcia-Fernandez J M, Provencio I, DeGrip W J. J Comp Physiol A. 1993;172:33–45. [Google Scholar]
- 34.Yoshikawa T, Yashiro Y, Oishi T, Kokame K, Fukada Y. Zool Sci. 1994;11:675–680. [PubMed] [Google Scholar]
- 35.Foster R G, Grace M S, Provencio I, Degrip W J, Garcia-Fernandez J M. Neurosci Biobehav Rev. 1994;18:541–546. doi: 10.1016/0149-7634(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 36.Steinach E. Arch Gesamte Physiol Menschen Tiere. 1892;52:495–525. [Google Scholar]
- 37.Barr L, Alpern M. J Gen Physiol. 1963;46:1249–1265. doi: 10.1085/jgp.46.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang M, Pandey S, Fong H K. Invest Ophthalmol Visual Sci. 1993;34:3669–3678. [PubMed] [Google Scholar]
- 39.Hui S, Gilbert D B, Copeland N G, Jenkins N A, Nathans J. Proc Natl Acad Sci USA. 1997;94:9893–9898. doi: 10.1073/pnas.94.18.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spudich J L, Lanyi J K. Curr Opin Cell Biol. 1996;8:452–457. doi: 10.1016/s0955-0674(96)80020-2. [DOI] [PubMed] [Google Scholar]
- 41.Deininger W, Kroger P, Hegemann U, Lottspeich F, Hegemann P. EMBO J. 1995;14:5849–5858. doi: 10.1002/j.1460-2075.1995.tb00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saranak J, Foster K W. Nature (London) 1997;387:465–466. doi: 10.1038/387465a0. [DOI] [PubMed] [Google Scholar]