Abstract
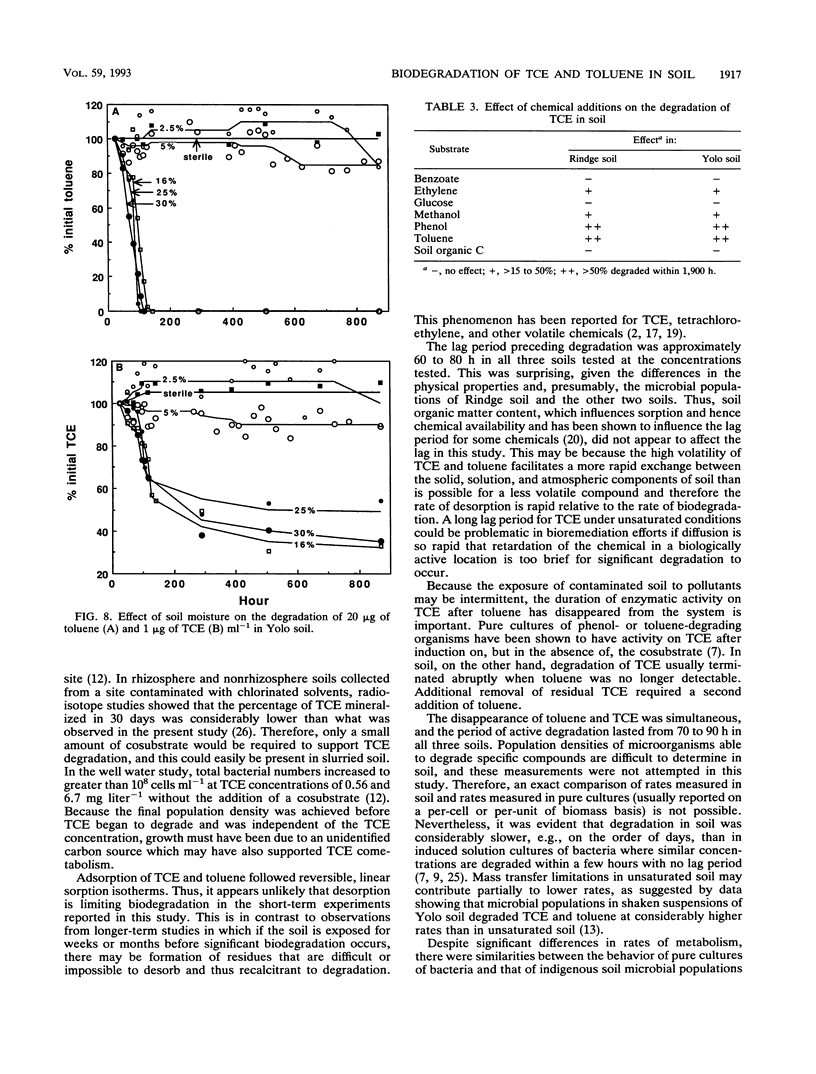
The biodegradation of trichloroethylene (TCE) and toluene, incubated separately and in combination, by indigenous microbial populations was measured in three unsaturated soils incubated under aerobic conditions. Sorption and desorption of TCE (0.1 to 10 micrograms ml-1) and toluene (1.0 to 20 micrograms ml-1) were measured in two soils and followed a reversible linear isotherm. At a concentration of 1 micrograms ml-1, TCE was not degraded in the absence of toluene in any of the soils. In combination, both 1 microgram of TCE ml-1 and 20 micrograms of toluene ml-1 were degraded simultaneously after a lag period of approximately 60 to 80 h, and the period of degradation lasted from 70 to 90 h. Usually 60 to 75% of the initial 1 microgram of TCE ml-1 was degraded, whereas 100% of the toluene disappeared. A second addition of 20 micrograms of toluene ml-1 to a flask with residual TCE resulted in another 10 to 20% removal of the chemical. Initial rates of degradation of toluene and TCE were similar at 32, 25, and 18 degrees C; however, the lag period increased with decreasing temperature. There was little difference in degradation of toluene and TCE at soil moisture contents of 16, 25, and 30%, whereas there was no detectable degradation at 5 and 2.5% moisture. The addition of phenol, but not benzoate, stimulated the degradation of TCE in Rindge and Yolo silt loam soils, methanol and ethylene slightly stimulated TCE degradation in Rindge soil, glucose had no effect in either soil, and dissolved organic carbon extracted from soil strongly sorbed TCE but did not affect its rate of biodegradation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abril M. A., Michan C., Timmis K. N., Ramos J. L. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J Bacteriol. 1989 Dec;171(12):6782–6790. doi: 10.1128/jb.171.12.6782-6790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign S. A., Hyman M. R., Arp D. J. Cometabolic degradation of chlorinated alkenes by alkene monooxygenase in a propylene-grown Xanthobacter strain. Appl Environ Microbiol. 1992 Sep;58(9):3038–3046. doi: 10.1128/aem.58.9.3038-3046.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensley B. D. Biochemical diversity of trichloroethylene metabolism. Annu Rev Microbiol. 1991;45:283–299. doi: 10.1146/annurev.mi.45.100191.001435. [DOI] [PubMed] [Google Scholar]
- Folsom B. R., Chapman P. J., Pritchard P. H. Phenol and trichloroethylene degradation by Pseudomonas cepacia G4: kinetics and interactions between substrates. Appl Environ Microbiol. 1990 May;56(5):1279–1285. doi: 10.1128/aem.56.5.1279-1285.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harker A. R., Kim Y. Trichloroethylene degradation by two independent aromatic-degrading pathways in Alcaligenes eutrophus JMP134. Appl Environ Microbiol. 1990 Apr;56(4):1179–1181. doi: 10.1128/aem.56.4.1179-1181.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C. D., Palumbo A. V., Herbes S. E., Lidstrom M. E., Tyndall R. L., Gilmer P. J. Trichloroethylene biodegradation by a methane-oxidizing bacterium. Appl Environ Microbiol. 1988 Apr;54(4):951–956. doi: 10.1128/aem.54.4.951-956.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. J., Montgomery S. O., Mahaffey W. R., Pritchard P. H. Biodegradation of trichloroethylene and involvement of an aromatic biodegradative pathway. Appl Environ Microbiol. 1987 May;53(5):949–954. doi: 10.1128/aem.53.5.949-954.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. J., Montgomery S. O., O'neill E. J., Pritchard P. H. Aerobic metabolism of trichloroethylene by a bacterial isolate. Appl Environ Microbiol. 1986 Aug;52(2):383–384. doi: 10.1128/aem.52.2.383-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. J., Montgomery S. O., Pritchard P. H. Trichloroethylene metabolism by microorganisms that degrade aromatic compounds. Appl Environ Microbiol. 1988 Feb;54(2):604–606. doi: 10.1128/aem.54.2.604-606.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C. R., McConnell G. Chlorinated C1 and C2 hydrocarbons in the marine environment. Proc R Soc Lond B Biol Sci. 1975 May 20;189(1096):305–332. doi: 10.1098/rspb.1975.0059. [DOI] [PubMed] [Google Scholar]
- Vogel T. M., McCarty P. L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985 May;49(5):1080–1083. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Gibson D. T. Degradation of trichloroethylene by toluene dioxygenase in whole-cell studies with Pseudomonas putida F1. Appl Environ Microbiol. 1988 Jul;54(7):1703–1708. doi: 10.1128/aem.54.7.1703-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton B. T., Anderson T. A. Microbial degradation of trichloroethylene in the rhizosphere: potential application to biological remediation of waste sites. Appl Environ Microbiol. 1990 Apr;56(4):1012–1016. doi: 10.1128/aem.56.4.1012-1016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]



