Abstract
Metallothionein (MT) has several putative roles in metal detoxification, in Zn and Cu homeostasis, in scavenging free radicals, and in the acute phase response. Mice of mixed 129/Ola and C57BL/6J background with targeted disruption of MT-I and MT-II genes are more sensitive to toxic metals and oxidative stress. We noted that these animals were larger than most strains of mice, and we systematically studied aspects of their physiology and biochemistry relating to energy metabolism. During the first 2 weeks after weaning, the growth rates of MT-null and C57BL/6J mice were similar, but the transgenic mice became significantly heavier at age 5–6 weeks. At age 14 weeks, the body weight and food intake of MT-null mice was 16 and 30% higher, respectively, compared with C57BL/6J mice. Most 22- to 39-week-old male MT-null mice were obese, as shown by increased fat accretion, elevated obese (ob) gene expression, and high plasma leptin levels, similar to those recorded in Zucker fatty (fa/fa) rats. Seven-week-old MT-null mice also had significantly higher levels of plasma leptin and elevated expression of ob, lipoprotein lipase, and CCAAT enhancer binding protein α genes as compared with age-matched C57BL/6J mice. These observations indicate that abnormal accretion of body fat and adipocyte maturation is initiated at 5–7 weeks of age, possibly coincident with sexual maturation. Targeted disruption of MT-I and MT-II genes seems to induce moderate obesity, providing a new obese animal model. A link between MT and the regulation of energy balance is implied.
The generation of transgenic mice with targeted gene disruption is very valuable in the study of the physiological function of specific proteins. Nevertheless, a surprising number of such animals show no apparent phenotypic abnormalities and procreate normally. For example, glutathione peroxidase is an important antioxidant, yet cellular glutathione peroxidase-null mice are not apparently disadvantaged in terms of their capacity to resist hyperoxia (1). Metallothioneins comprise a family of highly conserved low Mr metal-binding proteins that are reported to function in the detoxification of heavy metals, in Zn and Cu homeostasis, in the scavenging of free radicals, and in the acute phase response (2–4). However, mice with disrupted MT-I and -II genes are apparently phenotypically normal with no adverse effect on their ability to reproduce and rear offspring (5, 6). Nevertheless, they are more sensitive to metal and oxidant toxicity (5–9), and cells from such animals are less viable than equivalent cells from animals expressing MT-I and -II, when cultured in the presence of various toxic agents. (10, 11).
However, we have noted that mature animals from our colony of MT-null mice with a mixed 129/Ola–C57BL/6J background (5) generally attain higher body mass with larger reserves of fat than C57BL/6J mice. Abnormal energy balance was indicated, and a key link in the feedback control of appetite and energy expenditure is the protein leptin, a product of obese (ob) gene expression in white adipose tissue (12–14). Secreted into the plasma, leptin exerts its biological activity by binding to receptors, particularly in the hypothalamus. Defects in leptin expression or activity can result in obesity, for example in mice with a double recessive mutation of the ob gene (ob/ob mice), which do not produce functional leptin (15), or in Zucker fatty (fa/fa) rats, which have a defective leptin receptor protein (14). In both cases, ob gene expression is elevated, and the animals are hyperphagic, but only Zucker (fa/fa) rats have high levels of circulating leptin (14). Injection of ob/ob mice with recombinant murine leptin reduces food intake, increases energy expenditure, and decreases body weight (16–18).
To evaluate the prevalence of obesity in our MT-null mice, we have systematically studied their growth, food intake, lipid accumulation, the expression of ob gene in white adipose tissue, plasma leptin levels, and other indicators of energy metabolism.
MATERIALS AND METHODS
Animals.
Mice were produced with targeted disruption of both MT-I and MT-II genes (5). To establish a colony at the Rowett Institute, MT-null mice obtained from the conventional animal facility at the Murdoch Institute were rederived by sterile embryo transfer to isolator-reared surrogate mothers. The offspring tested negative to all common viral and bacterial mouse pathogens, and the resulting colony was maintained in a minimal disease unit. C57BL/6J mice of similar health status obtained from a commercial supplier (Harlan, Bicester, U.K.) were used as controls. Male mice were maintained for periods up to 1 year at an environmental temperature of 23°C with a 12-h light/dark cycle. A weight survey of the older MT-null male mouse population (58 mice aged 22–39 weeks) gave a mean weight of 40.3 ± SD 6.9 g (range, 28–59 g) with 25, 53, and 22% of animals in weight categories <36 g, 36–46 g, and >46 g, respectively.
Growth and Food Intake Study.
All mice were given a commercial pelleted mouse diet (CRM Diet, containing 2.4% oil, 18.1% protein, 57% carbohydrate, and 3.6% fiber, in addition to essential minerals, vitamins, and amino acids; Labsure, Poole, UK) and water ad libitum. The growth rate of male MT-null and C57BL/6J mice was monitored from weaning for an 11-week period. At 14 weeks of age, food intake by MT-null and C57BL/6J mice was monitored for a 17-day period. The animals were placed on grid-based cages, and spilled food falling through the grid was weighed and used to correct the calculation of food consumed from the difference in hopper food weight.
Physiological and Biochemical Studies.
In the first study, a group of 6-week-old C57BL/6J male mice were acclimated for 7 days to the same environmental conditions as a group of MT-null mice of the same age. All mice (10 in each group) were individually housed during the acclimation period and were given the same pelleted diet and water ad libitum.
In a second study, 18 MT-null mice aged 22–39 weeks were divided into three groups of six animals according to weight. In previous studies with MT-null mice (31), animals have been weight-matched with control mice, so for the purposes of comparison, one group (mean age 30 weeks, mean weight 32.0 ± SD 1.5 g) was weight-matched with the C57BL/6J control mice (31.6 ± SD 0.9 g) of similar age, which had been housed at the Rowett Institute for 22 weeks. Thus, the MT-null mice in this group were age-matched and weight-matched with the control group. However, as the mean weight for the MT-null mice was about 8 g higher than that of similarly aged control mice, a second group (32 weeks, 39.2 ± SD 2.5 g) of average weight for the MT-null mouse colony, aged 22–39 weeks, was formed. This group was therefore age-matched but not weight-matched with the C57BL/6J control mice. The fatty appearance of tissues in the larger MT-null mice of this age range had drawn our attention to the possibility of an obese phenotype in the colony, so we formed a third group of apparently obese animals (32 weeks, 50.4 ± SD 4.3 g). Lean, average weight, and obese MT-null mouse groups are hereafter referred to as groups Ln, Ave, and Obs.
Tissue and Blood Sample Collection.
Where possible, individual animals were removed from each group sequentially to eliminate any time-related influence on measured biochemical parameters. Blood was removed from the dorsal vena cava of mice under terminal anesthesia using heparinized syringes and transferred to microcentrifuge tubes on ice. The tubes were then centrifuged at 3500 × g for 10 min to obtain plasma, which was frozen and preserved at −80°C. Epididymal white adipose tissue (eWAT) and liver were removed, weighed, immediately frozen in liquid nitrogen, and then stored at −80°C until required.
Liver Glycogen, Lipid, Zn, Cu, and DNA.
Glycogen was extracted and analyzed by the method of Siu Lo et al. (19) using 50-mg liver samples, and total hepatic lipid was extracted and weighed according to the method of Folch et al. (20). A micro acid digestion technique in combination with atomic absorption spectrophotometry was used for Zn and Cu analysis (21). DNA was extracted from 50 mg of liver by homogenization in 1 ml of 2% perchloric acid followed by centrifugation at 15,000 × g for 15 min (4°C). The pellet was washed with 2% perchloric acid and then resuspended and incubated in 1 ml of 0.3 M sodium hydroxide for 1 h at 37°C. After addition of 400 μl of 2 M perchloric acid, the samples were recentrifuged, the pellet was resuspended in 1 ml of 0.5 M perchloric acid, and this solution was first incubated for 1 h at 4°C and then heat extracted at 70°C for a further 1 h. After recentrifugation, 200 μl of the supernatant was removed for assay of DNA by using the method of Burton (22).
White Adipose Tissue ob, Lipoprotein Lipase (LPL), and CCAAT Enhancer Binding Protein α (C/EBP α) Gene Expression.
The procedure for RNA extraction and Northern blotting was as previously described (23). Measurement of mRNA was made by hybridization with specific digoxigenin 5′end-labeled oligodeoxynucleotide probes and chemiluminescence detection. The probes used for ob and LPL mRNA have been described (23, 24) as has that for 18 S rRNA (23), which was used to correct for variations in the loading of RNA onto agarose gels and transfer to nylon membranes. A 20-mer oligodeoxynucleotide (5′-GGGCCGCGGCTCCACCTCGT-3′) for mouse C/EBP α mRNA detection was selected from the coding region of the published DNA sequence (25) (GenBank database accession no. M62362).
Immunoassay of MT-1, Insulin, and Leptin.
Hepatic MT-1 was assayed in triplicate by RIA using a rodent-specific sheep antiserum and rat MT-1 standards (26). Plasma leptin was measured by an ELISA (27) using a rabbit anti-mouse leptin antiserum with standards of mouse recombinant leptin (PeproTech, London, U.K.) and duplicate plasma samples of 100 μl. Insulin was measured in duplicate plasma samples of 50 μl by using an anti-porcine insulin antiserum and porcine insulin standards (28).
Statistical Analysis.
Unpaired Student’s t-tests were used for statistical comparison of the C57BL/6J and MT-null mouse groups from study 1, whereas data from study 2 were first analyzed by a one-way analysis of variance with subsequent group comparisons by using 1- or 2-tailed t-tests, as appropriate, and a pooled estimate of error. The graphics program origin (Microcal Software Inc., Northampton, MA) was used for linear regression and associated statistics.
RESULTS
Growth and Food Intake.
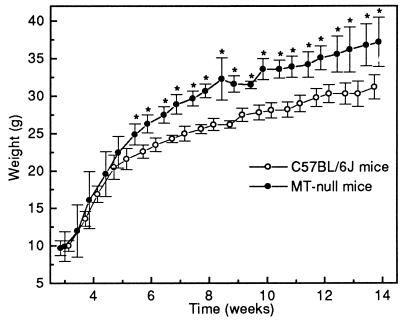
The growth rates of MT-null and C57BL/6J mice were similar from weaning until 5 weeks of age. However, MT-null mice became significantly and increasingly heavier during the following 3 weeks (Fig. 1). Between 8 and 14 weeks the growth rates of both groups were similar, and the MT-null mice remained heavier than the controls. Food intake by the MT-null mice from weeks 14–17 (6.1 ± SD 1.0 g/mouse/day) was significantly greater (P < 0.001) than that in the control group (4.5 ± SD 0.3 g/mouse/day).
Figure 1.
Growth curves of MT-null mice and C57BL/6J control mice from weaning (3 weeks) until 14 weeks of age. Each data point is the mean ± SD for five animals, and asterisks indicate statistical significance (P < 0.05), as indicated from t tests based on a pooled estimate of error.
Body Tissue Characteristics.
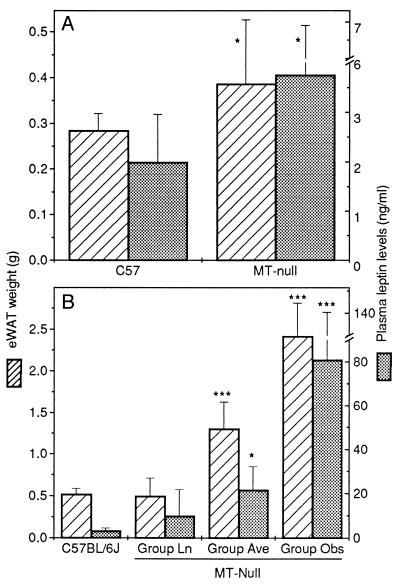
At termination of study 1, mean weights for C57BL/6J and MT-null mice were 22.7 ± SD 1.6 g and 25.6 ± SD 1.9 g, respectively, a difference that was statistically significant (P = 0.002). The eWAT weight of 7-week-old MT-null mice was significantly greater than that of control mice (Fig. 2A). In study 2 with mice aged 22–39 weeks, eWAT weight (Fig. 2B) and eWAT/body weight ratio (Table 1) in groups Ave and Obs were significantly greater than those in the control group. Liver weight in the group Obs was significantly higher than that in the control group, due in part to increased dry matter content (Table 1). However, liver weight, as a proportion of body weight, was consistently lower in all MT-null groups (Table 1).
Figure 2.
Epididymal white adipose tissue (eWAT) weight and plasma leptin levels in MT-null and C57BL/6J mice aged 7 weeks (A, study 1) and 22–39 weeks (B, study 2). MT-null mice in study 2 were divided into three weight groups: group Ln (body weight, 32.0 ± SD 1.5 g), which were weight-matched with the C57BL/6J group (31.6 ± SD 0.9 g), group Ave (39.2 ± SD 2.5 g), which were of average weight for the male MT-null mouse colony >22 weeks of age, and group Obs, which were apparently obese (50.4 ± SD 4.3 g). Error bars indicate the mean ± SD for six mice, and asterisks indicate statistically significant differences (∗, P < 0.05; ∗∗∗, P < 0.001) between MT-null groups and the C57BL/6J group.
Table 1.
Comparison of various physical and biochemical parameters in 22- to 39-week-old MT-null and C57BL/6J mice from study 2
| Parameter | C57BL/6J | Group Ln | Group Ave | Group Obs |
|---|---|---|---|---|
| eWAT/body weight ratio, mg/g | 16 ± 3 | 15 ± 6 | 33* ± 7 | 48*† ± 6 |
| Liver wet weight, g | 1.50 ± 0.10 | 1.31 ± 0.08 | 1.58 ± 0.20 | 1.91*† ± 0.41 |
| Liver dry/wet weight ratio, % | 27.8 ± 1.2 | 28.6 ± 1.1 | 28.5 ± 1.2 | 33.8*† ± 2.9 |
| Liver/body weight ratio, mg/g | 48 ± 2 | 41 ± 3 | 40* ± 3 | 38* ± 7 |
| Liver MT-I, μg/g wet weight | 9.10 ± 0.60 | 0.60* ± 0.14 | 0.36* ± 0.06 | 0.47* ± 0.09 |
| Liver Zn, μg/g dry weight | 94.7 ± 14.7 | 99.6 ± 6.0 | 97.3 ± 5.1 | 94.3 ± 11.3 |
| Liver Cu, μg/g dry weight | 20.0 ± 2.0 | 14.5* ± 1.2 | 15.3* ± 3.1 | 14.6* ± 2.7 |
| Plasma insulin, ng/ml | 1.29 ± 0.29 | 0.52 ± 0.16 | 0.95 ± 0.35 | 2.18* ± 1.17 |
| Liver glycogen, mg/mg DNA | 10.9 ± 8.3 | 5.6 ± 3.2 | 6.1 ± 2.0 | 7.2 ± 4.6 |
| Liver lipid, mg/mg DNA | 22.0 ± 7.1 | 15.4 ± 7.2 | 17.1 ± 7.5 | 35.6*† ± 17.3 |
Values are the mean ± SD for six mice and statistical comparisons were made using t tests based on a pooled estimate of error.
* Significantly different from the C57BL/6J control group (P < 0.05).
Significantly different from all other MT-null groups (P < 0.05).
Liver MT-1, Zn, and Cu.
As expected, hepatic MT-1 levels in all MT-null mice from study 2 were very low compared with the control levels (Table 1). Liver Zn levels were similar in all groups, but liver Cu levels were significantly lower in all MT-null groups compared with the C57BL/6J control group (Table 1).
Plasma Insulin, Liver Glycogen, and Liver Lipid Content.
Mice in group Obs had significantly higher plasma insulin levels than control mice, but levels in groups Ave and Ln were not different (Table 1). However, when combining data from all three MT-null groups, insulin levels were significantly correlated with eWAT weight (r = 0.75, P < 0.001) and plasma leptin concentration (r = 0.66, P = 0.003). Glycogen concentration in the liver (Table 1) showed no difference between groups. Hepatic lipid concentration was significantly higher in group Obs and when combining all MT-null data, total hepatic lipid was significantly correlated with eWAT weight (r = 0.72, P < 0.001).
eWAT ob mRNA and Plasma Leptin.
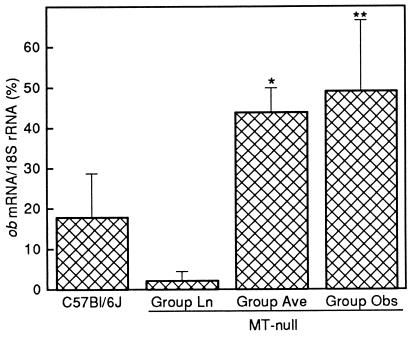
Compared with the controls, eWAT ob mRNA in 7-week-old MT-null mice (study 1) was significantly elevated (Fig. 3A), and this was associated with higher levels of circulating leptin (Fig. 2A). In the older animals of study 2, eWAT ob gene expression was significantly higher in groups Ave and Obs (Fig. 4), and plasma leptin levels were up to 25 times greater than those in control mice (Fig. 2B). The plasma leptin level of mice in group Ln was similar to that in the control group.
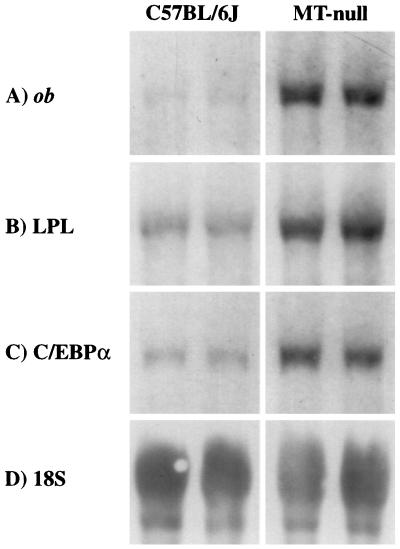
Figure 3.
Chemiluminescent detection of ob mRNA (A), LPL mRNA (B), C/EBP α mRNA (C), and 18S rRNA (D) on Northern blots of RNA from eWAT of 7-week-old MT-null and C57BL/6J mice (study 1) by using specific antisense digoxigenin-labeled oligonucleotides. Ten micrograms of total RNA was loaded onto each lane of the gel, and exposure of blots to film was 20–60 min at 37°C. Representative Northern blots of RNA from two animals in each group are shown.
Figure 4.
Levels of ob mRNA in eWAT of MT-null mice (study 2), weight categorized as described in the legend to Fig. 2, and also C57BL/6J control mice. Error bars indicate the mean ± SD for six mice, and asterisks indicate statistically significant differences (∗, P < 0.05; ∗∗, P < 0.01) between MT-null groups and the C57BL/6J group.
LPL mRNA and C/EBP α mRNA.
In addition to ob gene expression and plasma leptin levels, there are several other genes that are characteristically overexpressed in obesity, for example LPL in adipose tissue. LPL is required for the lipolysis of circulating triacylglycerol, an initial process in the release of fatty acids to facilitate their incorporation into tissues for utilization as an energy source or for storage following re-esterification. Mutant obese rodents, such as Zucker (fa/fa) rats, have high adipose tissue levels of LPL mRNA (29). The transcription factor, C/EBP α, promotes the terminal differentiation of adipocytes from pre-adipocytes (30) and is a useful indicator of adipocyte maturation. Both LPL and C/EBP α genes were also more highly expressed in eWAT from MT-null mice than from control animals (Fig. 3 B and C).
DISCUSSION
Hepatic MT-1 levels in all MT-null mice were much lower than those in the C57BL/6J control animals, thus confirming their MT-deficient status. In contrast to previous reports that MT-null transgenic mice are phenotypically normal (5, 6), the majority of adult male mice in our MT-null colony show moderate obesity. This was indicated by higher body and eWAT weights, with a higher eWAT/body weight ratio in both young and older adult males. In addition, both eWAT ob gene expression and plasma leptin levels were significantly higher than in control mice, as has been found in other mutant animal models of obesity. Indeed, it is remarkable that MT-null mice in group Ave had plasma leptin levels similar to those previously recorded in 12-week-old Zucker fatty rats (27). The highest level detected in C57BL/6J mice from studies 1 and 2 was almost 5 ng/ml, but we calculated that this level was exceeded in over 94% of all MT-null male mice in the colony aged 22–39 weeks.
As expected from the fatty appearance of their livers, hepatic lipid concentration in group Obs was significantly higher than that of the other groups. Indeed, lipid accounted for 43% of the difference in liver dry weight between the control group and group Obs. Plasma triacylglycerol levels of MT-null and C57BL/6J mice were, however, not significantly different (data not shown). It is surprising that these increases in body weight and WAT content of MT-null mice have not been reported previously. However, Philcox et al. (31) did note that the proportion of eWAT to body weight tended to be higher in MT-null mice than in C57BL6 control animals of matched body weight (24 g) and that this difference became statistically significant after fasting. In a recent abstract (32), weanling MT-null mice fed diets containing 12.5 or 25 mg Zn/kg had a significantly higher body weight than 129/Sv control mice.
Growth rates of our C57BL/6J and MT-null mice were similar until around 5 weeks of age, when there was a marked retardation of weight gain in the control animals but not in the MT-null mice. Hence, the trend toward obesity seems to begin at around sexual maturity, which is 5–6 weeks of age in the C57BL/6J mice. The growth rate of adult MT-null male mice exceeds that of most in-bred or out-bred mice from commercial suppliers, and it seems that their extended growth period is more a consequence of fat deposition than of increased lean body mass. Nevertheless, preliminary data from our laboratory suggest that MT-null mice also have a higher lean body mass than age-matched C57BL/6J mice.
It is significant that gene expression of C/EBP α, a transcription factor that promotes adipocyte terminal differentiation (30) and up-regulates ob gene expression (33, 34), was increased in MT-null mice of age 7 weeks (Fig. 3) but not in the older mice (data not shown). Therefore, accretion of body fat at least partly accounts for the divergence of C57BL/6J and MT-null mouse weights and may be triggered by hormonal or biochemical changes associated with sexual maturation. The timing of puberty was not studied in the two mouse strains, but there is good evidence that maturation is related to body fat reserves (35), and leptin has been suggested as the primary or permissive signal initiating puberty (36, 37) through stimulation of gonadotropin release from the pituitary (38). Hence, leptin administered to female mice hastens the onset of reproductive function (37), and when administered to ob/ob mice, which are sterile, fertility is restored (39).
In rodent models of obesity, such as the ob/ob and diabetic (db/db) mutant mice, accretion of fat is caused by a combination of hyperphagia and reduced energy expenditure. Our MT-null mice, with established obesity, are hyperphagic, and their food intake is about 30% greater than that of their C57BL/6J counterparts. Thus, enhanced food intake seems to be important in maintaining the obese state of the animals and is possibly, at least in part, the cause of its development. Preliminary data from mature male mice suggest that food intake in the MT-null mice is not closely correlated with body weight.
Insulin administration to mice promotes ob gene expression (27) and may thereby help to regulate appetite. Conversely, injection of ob/ob mice with leptin markedly reduces the characteristically high circulating insulin levels (16), and the effectiveness of insulin on, for example, glucose transport in cultured adipocytes seems to be reduced in the presence of elevated leptin (40). In this study, plasma insulin levels in groups Ln and Ave from study 2 were not significantly different from control levels. This finding is similar to previous reports (41, 42). However, it was clear that group Obs had significantly higher levels of circulating insulin. Indeed, when combining the data for all MT-null mice, we found a highly significant positive correlation of eWAT weight (r = 0.751, P < 0.001) and plasma leptin (r = 0.659, P = 0.003) with plasma insulin. Concomitantly high levels of leptin and insulin in combination with elevated food intake could indicate insensitivity to both hormones and possibly stimulation of their expression by a common promoter such as corticosterone. In addition to other factors, glucocorticoids are known to stimulate adipose tissue LPL gene transcription, and eWAT LPL mRNA levels were significantly higher in 7-week-old MT-null mice than in the corresponding C57BL/6J control animals.
As reported previously (41, 42), plasma glucose levels of MT-null and C57BL/6J mice were similar; furthermore, there was no relationship between plasma glucose levels and either plasma insulin or leptin levels (data not shown). Rofe et al. (41) have observed that liver glycogen concentrations, as related to liver wet weight, are lower in MT-null mice than in C57BL6 mice. Our data indicated consistently decreased liver glycogen levels in all three MT-null mouse groups compared with the control group, but these differences were not statistically significant. The lack of MT in the liver did not markedly affect hepatic Zn levels, as has been shown previously (43), although hepatic Cu levels were reduced. Since Cu is not normally the dominant metal bound to MT, it seems unlikely that this reduction was due to lack of MT-related Cu binding capacity. Indeed, total liver Cu and Zn contents were the same in groups Ln and Ave and the control group but significantly higher in group Obs (data not shown).
From a mechanistic perspective, the key question is how the lack of MT-I and/or MT-II leads to obesity. There is good, if not always definitive, evidence supporting roles for MT in metal detoxification, in Zn and Cu homeostasis, in scavenging of free radicals, and in protecting against various stress factors. MT-null mice do seem to be more affected by certain toxic agents and stress factors (5, 6, 8, 9, 44–46), and this is particularly evident when cells are studied in primary culture (10, 11, 43). Systematic study of MT-null mice by others has failed to demonstrate any major and consistent disturbance in normal physiological processes.
The central finding of this study was the increase in plasma leptin levels in most MT-null mice, and it is possible that MT isoforms could directly or indirectly influence ob gene expression. Leptin signaling is mediated by the long form of a cytokine-like receptor, which is found particularly in the hypothalamus but also elsewhere, such as in hepatic parenchymal cells (47), testes, adrenal gland, and renal medulla (48, 49). If MT were to play a role in the chain of signaling events initiated by the binding of leptin to its receptor, the absence of MT might be expected to stimulate overcompensation of ob gene expression. Alternatively, MT could play a role in the feedback suppression of ob gene expression. That this occurs by endogenous expression of MT in eWAT seems very unlikely, however, as MT is not expressed in this tissue in response to known inducers of MT gene expression, such as Zn injection or cold exposure (21). However, the considerable cold-induced expression of MT-I in brown adipose tissue (21), which undergoes marked metabolic transformation during thermogenesis, could indicate a role of MT in the utilization of energy reserves. Although the expression of MT and of leptin is influenced in opposite ways by certain stress factors, such as cold exposure and starvation, coexpression can also occur, such as following infection or administration of glucocorticoids and their analogues. The body weight divergence between MT-null and C57BL/6J mice at sexual maturity indicates that steroid hormone metabolism at this stage in development could be affected by the lack of MT.
It is possible that the development of obesity and the associated biochemical changes in the MT-null mice are caused by factors other than lack of MT. For example, disruption of MT genes by homologous recombination with DNA containing various modifications may have affected other genes around this locus or had downstream effects on gene expression. Such consequences of targeted gene disruption have been noted previously (50).
In conclusion, we have demonstrated that the MT-null mice from our established colony become moderately obese at or before 7 weeks of age, as manifested by increases in body weight, white adipose tissue weight, expression of ob, LPL, and C/EBP α genes, and plasma concentrations of leptin. Older mice aged 22–39 weeks were even more obese with similar characteristics to other mutant animal models of obesity, such as the diabetic (db/db) mouse and the Zucker (fa/fa) rat. These results could imply a novel role for MT in the regulation of energy balance, but development of obesity as an indirect consequence of the targeted gene disruption used to produce the MT-null mice cannot yet be excluded.
Acknowledgments
We thank Mr. T. Atkinson for analysis of plasma insulin and also Mrs. D. Bourke and the Rowett Institute animal house staff for helpful advice and assistance with establishment and maintenance of the MT-null mouse colony. Useful discussions with Dr. A. K. West, Biochemistry Department, University of Tasmania, Hobart, Australia, are also appreciated. This work was funded by the Scottish Office Agriculture, Environment, and Fisheries Department, U.K.
ABBREVIATIONS
- MT
metallothionein
- C/EBP α
CCAAT enhancer binding protein
- LPL
lipoprotein lipase
- Ln
lean
- Ave
average
- Obs
obese
- eWAT
epididymal white adipose tissue
References
- 1.Ho Y S, Magnenat J L, Bronson R T, Cao J, Gargano M, Sugawara M, Funk C D. J Biol Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 2.Andrews G K. Prog Food Nutr Sci. 1990;14:193–258. [PubMed] [Google Scholar]
- 3.Bremner I, Beattie J H. Annu Rev Nutr. 1990;10:63–83. doi: 10.1146/annurev.nu.10.070190.000431. [DOI] [PubMed] [Google Scholar]
- 4.Beattie J H, Bremner I. In: Trace Elements in Man and Animals: 9. Proceedings of the Ninth International Symposium on Trace Elements in Man and Animals. Fischer P W F, L’Abbé M R, Cockell K A, Gibson R S, editors. Ottawa: NRC Research Press; 1997. pp. 367–371. [Google Scholar]
- 5.Michalska A E, Choo K H A. Proc Natl Acad Sci USA. 1993;90:8088–8092. doi: 10.1073/pnas.90.17.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masters B A, Kelly E J, Quaife C J, Brinster R L, Palmiter R D. Proc Natl Acad Sci USA. 1994;91:584–588. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Liu Y P, Michalska A E, Choo K H A, Klaassen C D. J Pharmacol Exp Ther. 1996;276:1216–1223. [PubMed] [Google Scholar]
- 8.Sato M, Apostolova M D, Hamaya M, Yamaki J, Choo K H A, Michalska A E, Kodama N, Tohyama C. Environ Toxicol Pharmacol. 1996;1:221–225. doi: 10.1016/1382-6689(96)00011-7. [DOI] [PubMed] [Google Scholar]
- 9.Kelly E J, Quaife C J, Froelick G J, Palmiter R D. J Nutr. 1996;126:1782–1790. doi: 10.1093/jn/126.7.1782. [DOI] [PubMed] [Google Scholar]
- 10.Kondo Y, Woo E S, Michalska A E, Choo K H A, Lazo J S. Cancer Res. 1995;55:2021–2023. [PubMed] [Google Scholar]
- 11.Lazo J S, Kondo Y, Dellapiazza D, Michalska A E, Choo K H A, Pitt B R. J Biol Chem. 1995;270:5506–5510. doi: 10.1074/jbc.270.10.5506. [DOI] [PubMed] [Google Scholar]
- 12.Flier J S. Proc Natl Acad Sci USA. 1997;94:4242–4245. doi: 10.1073/pnas.94.9.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf G. Nutr Rev. 1997;55:85–88. doi: 10.1111/j.1753-4887.1997.tb01602.x. [DOI] [PubMed] [Google Scholar]
- 14.White B D, Martin R J. Proc Soc Exp Biol Med. 1997;214:222–232. doi: 10.3181/00379727-214-44090. [DOI] [PubMed] [Google Scholar]
- 15.Weigle D S, Kuijper J L. BioEssays. 1996;18:867–874. doi: 10.1002/bies.950181105. [DOI] [PubMed] [Google Scholar]
- 16.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 17.Halaas J L, Gajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 18.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 19.Siu Lo J, Russell C, Taylor A W. J Appl Physiol. 1970;28:234–236. doi: 10.1152/jappl.1970.28.2.234. [DOI] [PubMed] [Google Scholar]
- 20.Folch J, Lees M, Sloane Stanley G H. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 21.Beattie J H, Black D J, Duncan J S, Wood A M, Trayhurn P. Am J Physiol. 1996;270:R971–R977. doi: 10.1152/ajpregu.1996.270.5.R971. [DOI] [PubMed] [Google Scholar]
- 22.Burton K. Biochem J. 1956;62:315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trayhurn P, Duncan J S, Rayner D V. Biochem J. 1995;311:729–733. doi: 10.1042/bj3110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trayhurn P, Duncan J S, Nestor A, Thomas M E, Rayner D V. Anal Biochem. 1994;222:224–230. doi: 10.1006/abio.1994.1477. [DOI] [PubMed] [Google Scholar]
- 25.Christy R J, Kaestner K H, Geiman D E, Lane M D. Proc Natl Acad Sci USA. 1991;88:2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehra R K, Bremner I. Biochem J. 1983;213:459–465. doi: 10.1042/bj2130459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardie L J, Rayner D V, Holmes S, Trayhurn P. Biochem Biophys Res Commun. 1996;223:660–665. doi: 10.1006/bbrc.1996.0951. [DOI] [PubMed] [Google Scholar]
- 28.Mercer J G, Lawrence C B, Atkinson T. Physiol Behav. 1996;60:121–127. doi: 10.1016/0031-9384(95)02255-4. [DOI] [PubMed] [Google Scholar]
- 29.Greenwood M R C, Cleary M, Steingrimsdottir L, Vasselli J R. In: Recent Advances in Obesity Research: III. Björntorp P, Cairella M, Howard A N, editors. London: John Libbey; 1981. pp. 75–79. [Google Scholar]
- 30.Umek R M, Friedman A D, McKnight S L. Science. 1991;251:288–292. doi: 10.1126/science.1987644. [DOI] [PubMed] [Google Scholar]
- 31.Philcox J C, Coyle P, Michalska A, Choo K H A, Rofe A M. Biochem J. 1995;308:543–546. doi: 10.1042/bj3080543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffy J Y, Baines D, Keen C L, Daston G P. Teratology. 1997;55:54. doi: 10.1002/(SICI)1096-9926(199711)56:5<327::AID-TERA6>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 33.He Y, Chen H, Quon M J, Reitman M. J Biol Chem. 1995;270:28887–28891. doi: 10.1074/jbc.270.48.28887. [DOI] [PubMed] [Google Scholar]
- 34.Hwang C S, Mandrup S, MacDougald O A, Geiman D E, Lane M D. Proc Natl Acad Sci USA. 1996;93:873–877. doi: 10.1073/pnas.93.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ronnekleiv O K, Ojeda S R, McCann S M. Biol Reprod. 1978;19:414–424. doi: 10.1095/biolreprod19.2.414. [DOI] [PubMed] [Google Scholar]
- 36.Cheung C C, Thornton J E, Kuijper J L, Weigle D S, Clifton D K, Steiner R A. Endocrinology. 1997;138:855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- 37.Chehab F F, Mounzih K, Lu R, Lim M E. Science. 1997;275:88–90. doi: 10.1126/science.275.5296.88. [DOI] [PubMed] [Google Scholar]
- 38.Yu W H, Kimura M, Walczewska A, Karanth S, McCann S M. Proc Natl Acad Sci USA. 1997;94:1023–1028. doi: 10.1073/pnas.94.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chehab F F, Lim M E, Lu R. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 40.Muller G, Ertl J, Gerl M, Preibisch G. J Biol Chem. 1997;272:10585–10593. doi: 10.1074/jbc.272.16.10585. [DOI] [PubMed] [Google Scholar]
- 41.Rofe A M, Philcox J C, Coyle P. Biochem J. 1996;314:793–797. doi: 10.1042/bj3140793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Apostolova M D, Choo K H A, Michalska A E, Tohyama C. J Trace Elem Med Biol. 1997;11:1–7. doi: 10.1016/S0946-672X(97)80001-6. [DOI] [PubMed] [Google Scholar]
- 43.Coyle P, Philcox J C, Rofe A M. Biochem J. 1995;309:25–31. doi: 10.1042/bj3090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng H, Liu J, Choo K H A, Michalska A E, Klaassen C D. Toxicol Appl Pharmacol. 1996;136:229–235. doi: 10.1006/taap.1996.0029. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Liu Y, Michalska A E, Choo K H A, Klaassen C D. J Pharmacol Exp Ther. 1996;276:1216–1223. [PubMed] [Google Scholar]
- 46.Kelly E J, Palmiter R D. Nat Genet. 1996;13:219–222. doi: 10.1038/ng0696-219. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Kuropatwinski K K, White D W, Hawley T S, Hawley R G, Tartaglia L A, Baumann H. J Biol Chem. 1997;272:16216–16223. doi: 10.1074/jbc.272.26.16216. [DOI] [PubMed] [Google Scholar]
- 48.Hoggard N, Mercer J G, Rayner D V, Moar K, Trayhurn P, Williams L M. Biochem Biophys Res Commun. 1997;232:383–387. doi: 10.1006/bbrc.1997.6245. [DOI] [PubMed] [Google Scholar]
- 49.Serradeil-Le Gal C, Raufaste D, Brossard G, Pouzet B, Marty E, Maffrand J P, Le Fur G. FEBS Lett. 1997;404:185–191. doi: 10.1016/s0014-5793(97)00125-7. [DOI] [PubMed] [Google Scholar]
- 50.Olson E N, Arnold H-H, Rigby P W J, Wold B J. Cell. 1996;85:1–4. doi: 10.1016/s0092-8674(00)81073-9. [DOI] [PubMed] [Google Scholar]