Abstract
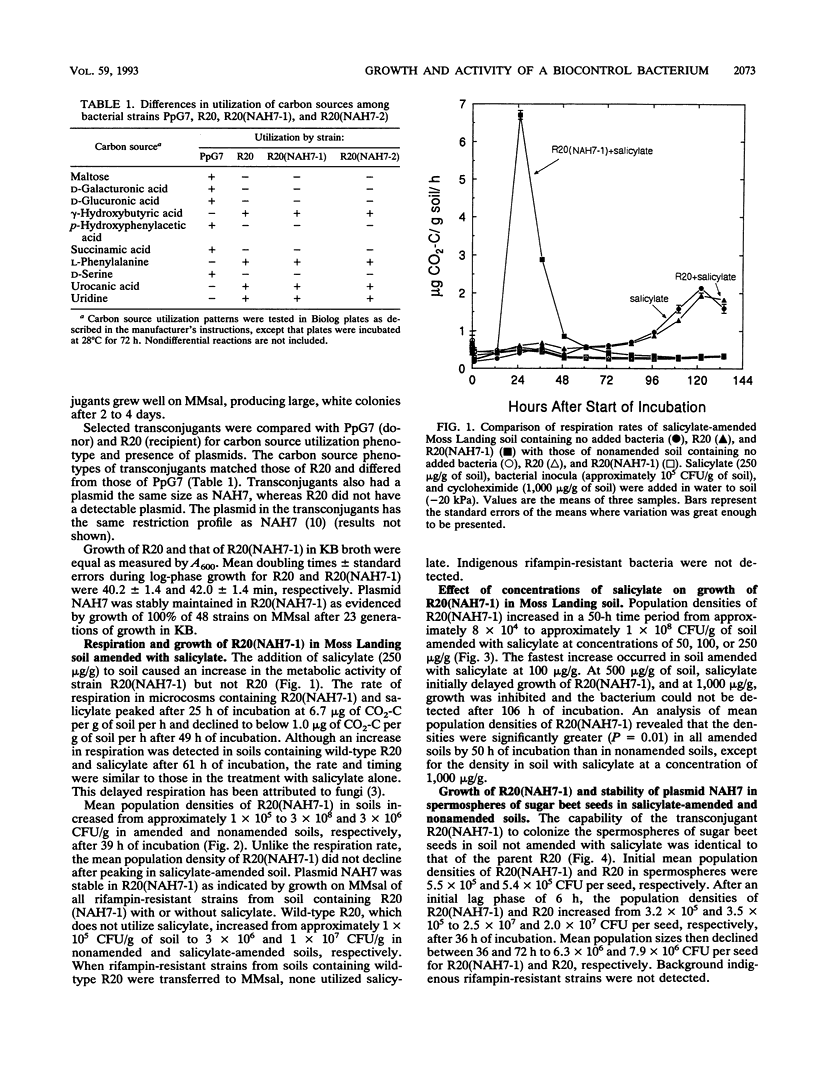
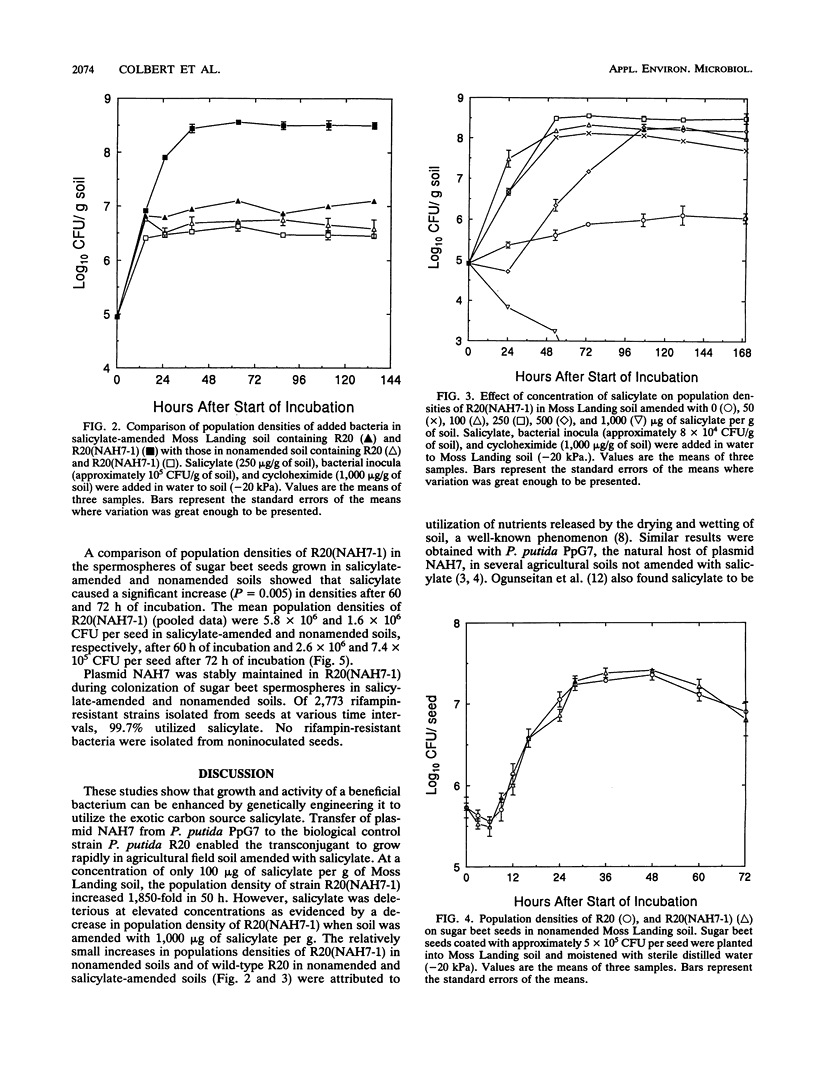
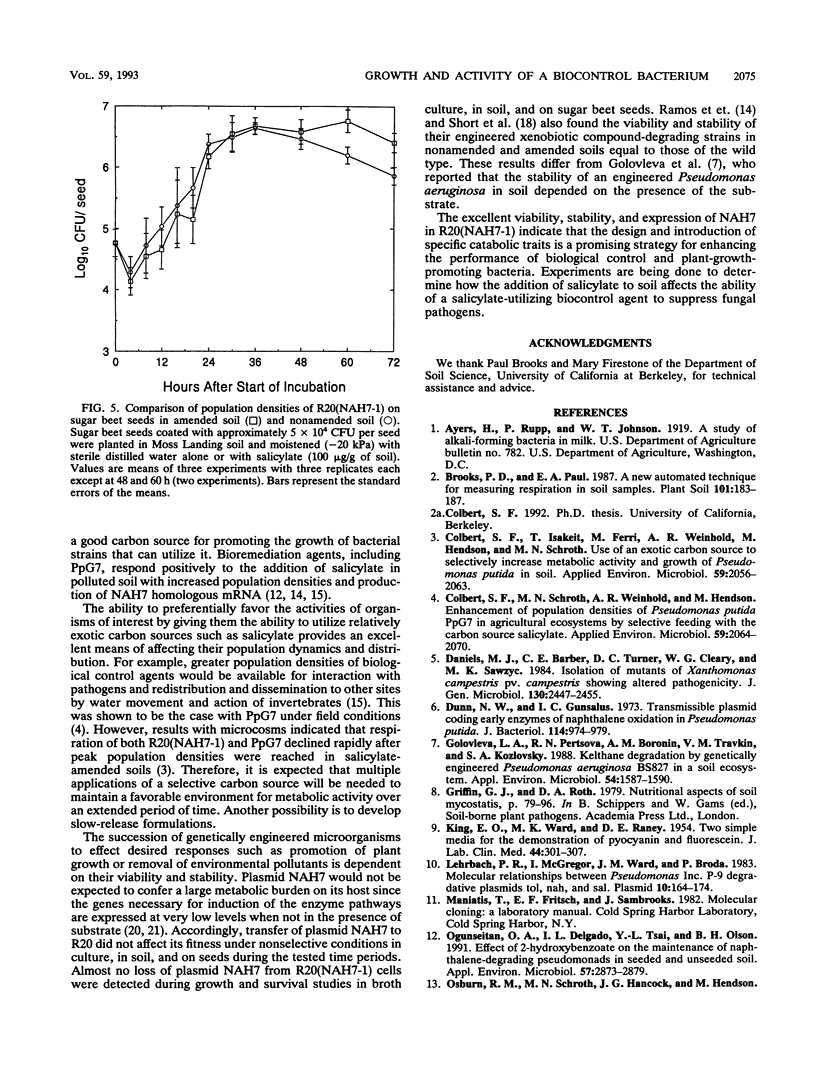
Plasmid NAH7 was transferred from Pseudomonas putida PpG7 to P. putida R20 [R20(NAH7)], an antagonist of Pythium ultimum. The plasmid did not affect growth or survival of R20(NAH7) and was stably maintained under nonselective conditions in broth and soil and on sugar beet seeds. Plasmid NAH7 conferred to R20(NAH7) the ability to utilize salicylate in culture, agricultural field soil, and on sugar beet seeds. The metabolic activity of R20(NAH7), but not the wild-type R20, was greatly increased in soil by amendment with salicylate (250 μg/g) as measured by induced respiration. Population densities of R20(NAH7) were also enhanced in salicylate-amended soil, increasing from approximately 1 × 105 CFU/g to approximately 3 × 108 CFU/g after 35 h of incubation. In contrast, population densities of R20(NAH7) in nonamended soil were approximately 3 × 106 CFU/g of soil after 35 h of incubation. The concentration of salicylate in soil affected the rate and extent of population increase by R20(NAH7). At 50 to 250 μg of salicylate per g of soil, population densities of R20(NAH7) increased to approximately 108 CFU/g of soil by 48 h of incubation, with the fastest increase at 100 μg/g. A lag phase of approximately 24 h occurred before the population density increased in the presence of salicylate at 500 μg/g; at 1,000 μg/g, population densities of R20(NAH7) declined over the time period of the experiment. Population densities of R20(NAH7) on sugar beet seeds in soils amended with 100 μg of salicylate per g were not increased while ample carbon was present in the spermosphere. However, after carbon from the seed had been utilized, population densities of R20(NAH7) decreased significantly less (P = 0.005) on sugar beet seeds in soil amended with salicylate than in nonamended soil.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colbert S. F., Isakeit T., Ferri M., Weinhold A. R., Hendson M., Schroth M. N. Use of an Exotic Carbon Source To Selectively Increase Metabolic Activity and Growth of Pseudomonas putida in Soil. Appl Environ Microbiol. 1993 Jul;59(7):2056–2063. doi: 10.1128/aem.59.7.2056-2063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn N. W., Gunsalus I. C. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J Bacteriol. 1973 Jun;114(3):974–979. doi: 10.1128/jb.114.3.974-979.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovleva L. A., Pertsova R. N., Boronin A. M., Travkin V. M., Kozlovsky S. A. Kelthane degradation by genetically engineered Pseudomonas aeruginosa BS827 in a soil ecosystem. Appl Environ Microbiol. 1988 Jun;54(6):1587–1590. doi: 10.1128/aem.54.6.1587-1590.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Lehrbach P. R., McGregor I., Ward J. M., Broda P. Molecular relationships between pseudomonas INC P-9 degradative plasmids TOL, NAH, and SAL. Plasmid. 1983 Sep;10(2):164–174. doi: 10.1016/0147-619x(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Ogunseitan O. A., Delgado I. L., Tsai Y. L., Olson B. H. Effect of 2-hydroxybenzoate on the maintenance of naphthalene-degrading pseudomonads in seeded and unseeded soil. Appl Environ Microbiol. 1991 Oct;57(10):2873–2879. doi: 10.1128/aem.57.10.2873-2879.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J. L., Duque E., Ramos-Gonzalez M. I. Survival in soils of an herbicide-resistant Pseudomonas putida strain bearing a recombinant TOL plasmid. Appl Environ Microbiol. 1991 Jan;57(1):260–266. doi: 10.1128/aem.57.1.260-266.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K. M., Gunsalus I. C. Plasmid gene organization: naphthalene/salicylate oxidation. Proc Natl Acad Sci U S A. 1982 Feb;79(3):874–878. doi: 10.1073/pnas.79.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen K. M., Serdar C. M. Genetics of naphthalene catabolism in pseudomonads. Crit Rev Microbiol. 1988;15(3):247–268. doi: 10.3109/10408418809104459. [DOI] [PubMed] [Google Scholar]



