Abstract
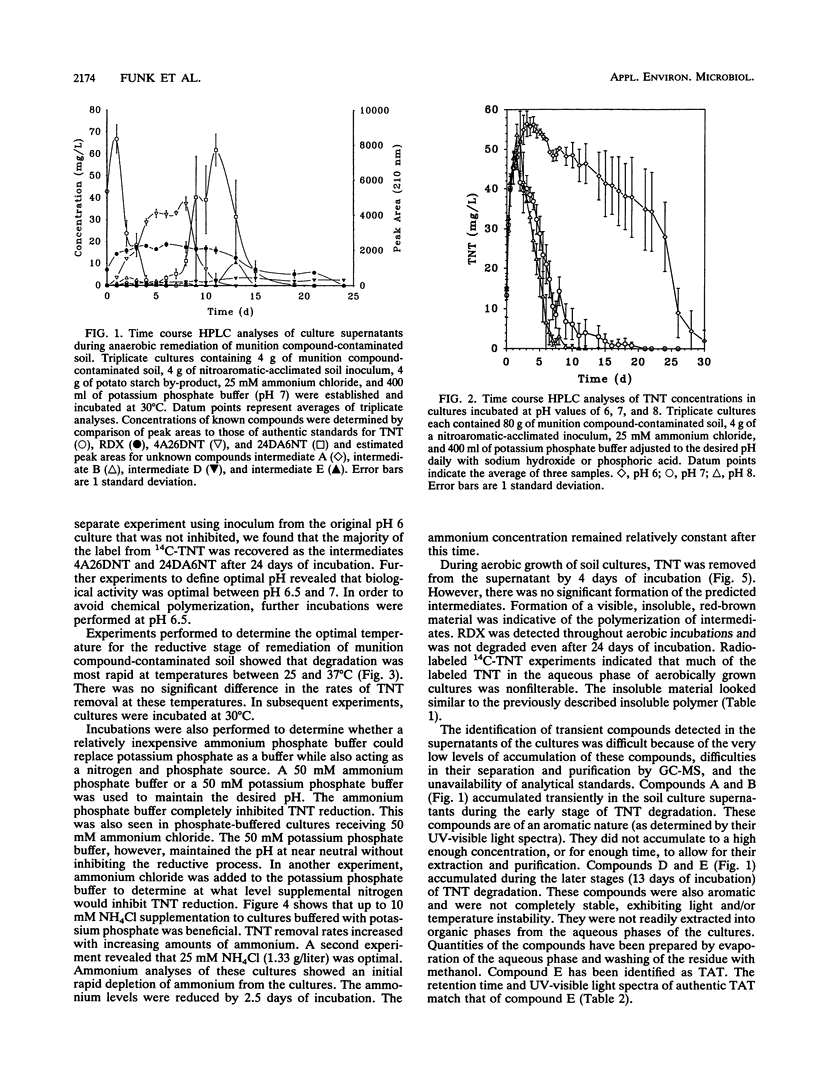
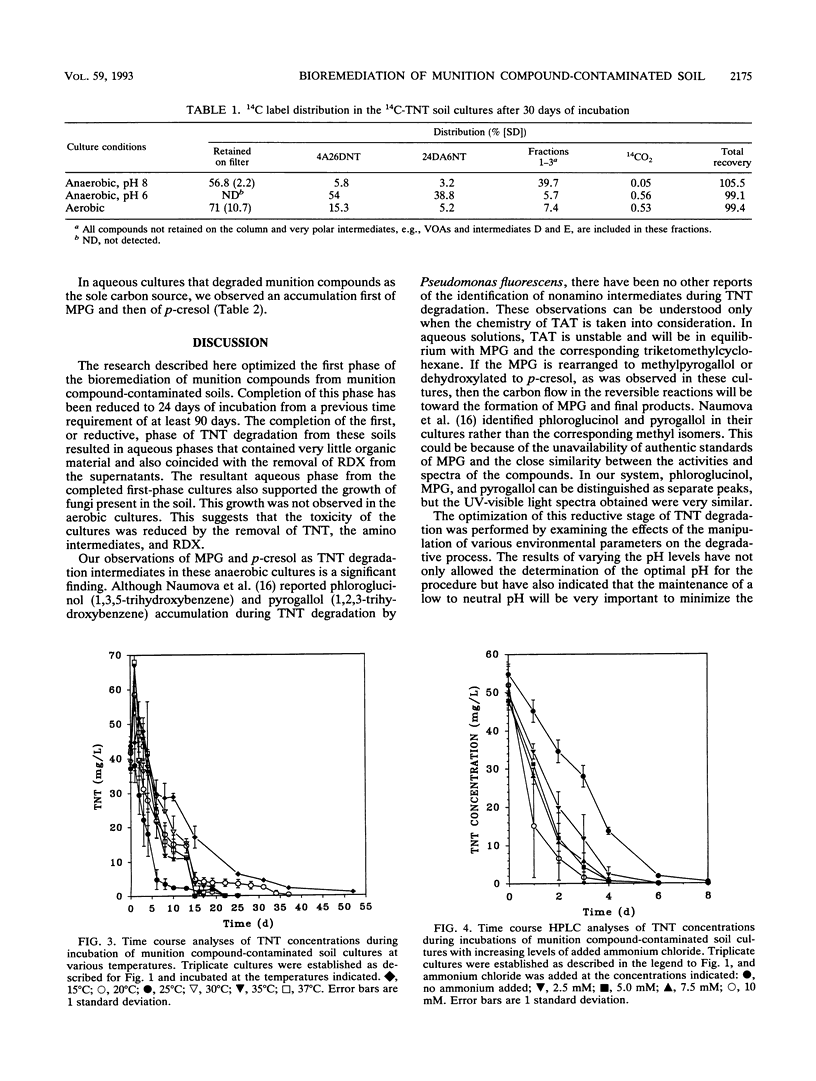
We examined the bioremediation of soils contaminated with the munition compounds 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-1,3,5-triazine, and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetraazocine by a procedure that produced anaerobic conditions in the soils and promoted the biodegradation of nitroaromatic contaminants. This procedure consisted of flooding the soils with 50 mM phosphate buffer, adding starch as a supplemental carbon substrate, and incubating under static conditions. Aerobic heterotrophs, present naturally in the soil or added as an inoculum, quickly removed the oxygen from the static cultures, creating anaerobic conditions. Removal of parent TNT molecules from the soil cultures by the strictly anaerobic microflora occurred within 4 days. The reduced intermediates formed from TNT and hexahydro-1,3,5-trinitro-1,3,5-triazine were removed from the cultures within 24 days, completing the first stage of remediation. The procedure was effective over a range of incubation temperatures, 20 to 37 degrees C, and was improved when 25 mM ammonium was added to cultures buffered with 50 mM potassium phosphate. Ammonium phosphate buffer (50 mM), however, completely inhibited TNT reduction. The optimal pH for the first stage of remediation was between 6.5 and 7.0. When soils were incubated under aerobic conditions or under anaerobic conditions at alkaline pHs, the TNT biodegradation intermediates polymerized. Polymerization was not observed at neutral to slightly acidic pHs under anaerobic conditions. Completion of the first stage of remediation of munition compound-contaminated soils resulted in aqueous supernatants that contained no munition residues or aminoaromatic compounds.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boopathy R., Kulpa C. F. Trinitrotoluene (TNT) as a sole nitrogen source for a sulfate-reducing bacterium Desulfovibrio sp. (B strain) isolated from an anaerobic digester. Curr Microbiol. 1992 Oct;25(4):235–241. doi: 10.1007/BF01570724. [DOI] [PubMed] [Google Scholar]
- Carpenter D. F., McCormick N. G., Cornell J. H., Kaplan A. M. Microbial transformation of 14C-labeled 2,4,6-trinitrotoluene in an activated-sludge system. Appl Environ Microbiol. 1978 May;35(5):949–954. doi: 10.1128/aem.35.5.949-954.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando T., Bumpus J. A., Aust S. D. Biodegradation of TNT (2,4,6-trinitrotoluene) by Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Jun;56(6):1666–1671. doi: 10.1128/aem.56.6.1666-1671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaake R. H., Roberts D. J., Stevens T. O., Crawford R. L., Crawford D. L. Bioremediation of soils contaminated with the herbicide 2-sec-butyl-4,6-dinitrophenol (dinoseb). Appl Environ Microbiol. 1992 May;58(5):1683–1689. doi: 10.1128/aem.58.5.1683-1689.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. L., Kaplan A. M. Thermophilic biotransformations of 2,4,6-trinitrotoluene under simulated composting conditions. Appl Environ Microbiol. 1982 Sep;44(3):757–760. doi: 10.1128/aem.44.3.757-760.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumova R. P., Selivanovskaia S. Iu, Mingatina F. A. Izuchenie vozmozhnosti glubokoi bakterial'noi destruktsii 2,4,6-trinitrotoluola. Mikrobiologiia. 1988 Mar-Apr;57(2):218–222. [PubMed] [Google Scholar]
- Roberts D. J., Kaake R. H., Funk S. B., Crawford D. L., Crawford R. L. Anaerobic remediation of dinoseb from contaminated soil. An on-site demonstration. Appl Biochem Biotechnol. 1993 Spring;39-40:781–789. doi: 10.1007/BF02919035. [DOI] [PubMed] [Google Scholar]
- Spiker J. K., Crawford D. L., Crawford R. L. Influence of 2,4,6-trinitrotoluene (TNT) concentration on the degradation of TNT in explosive-contaminated soils by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1992 Sep;58(9):3199–3202. doi: 10.1128/aem.58.9.3199-3202.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


