Abstract
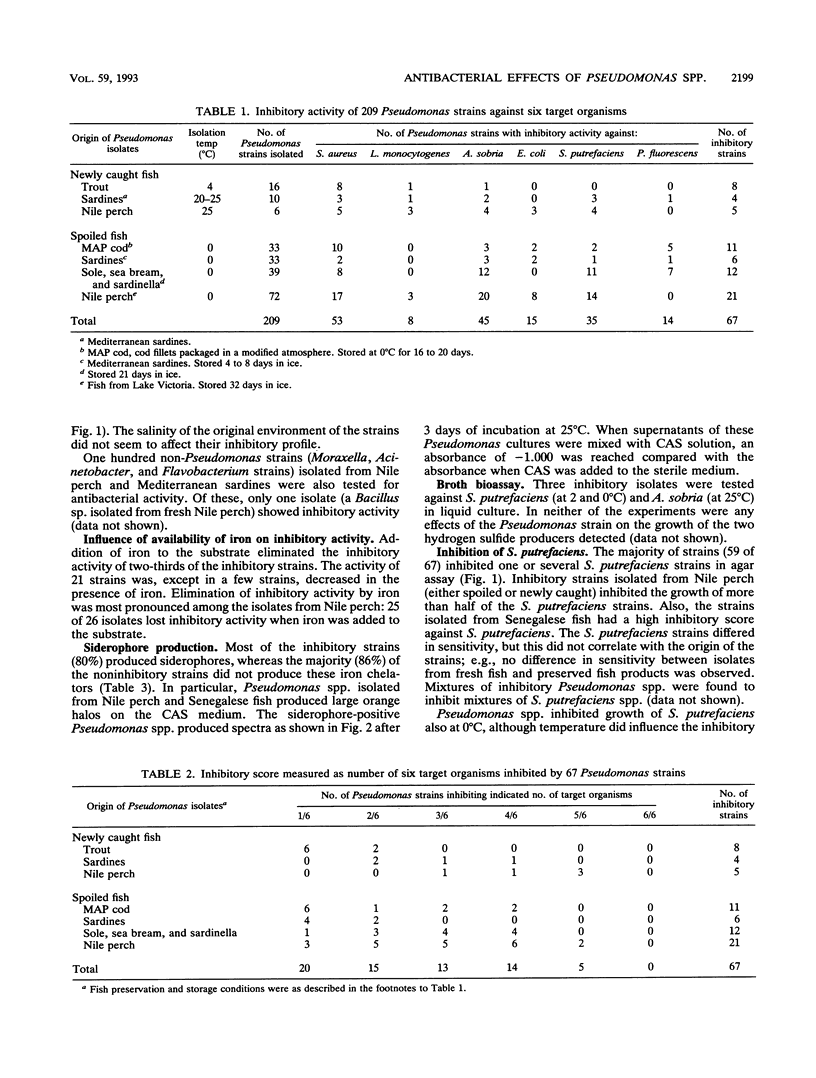
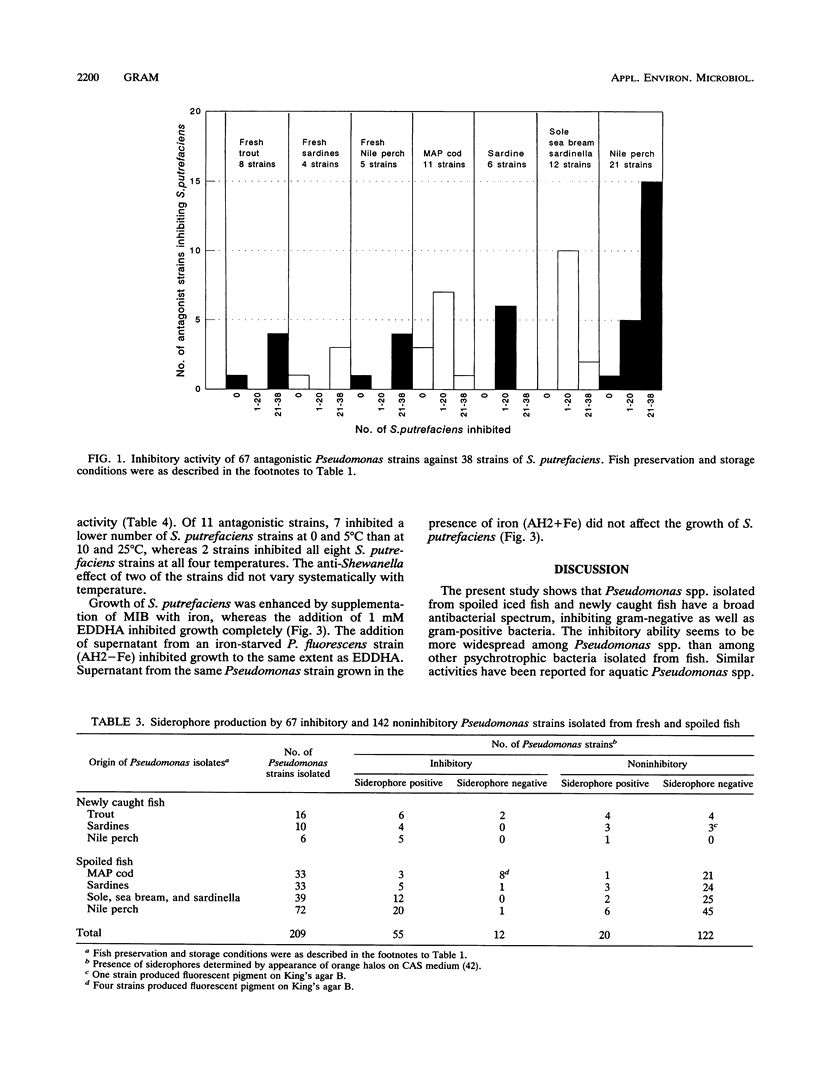
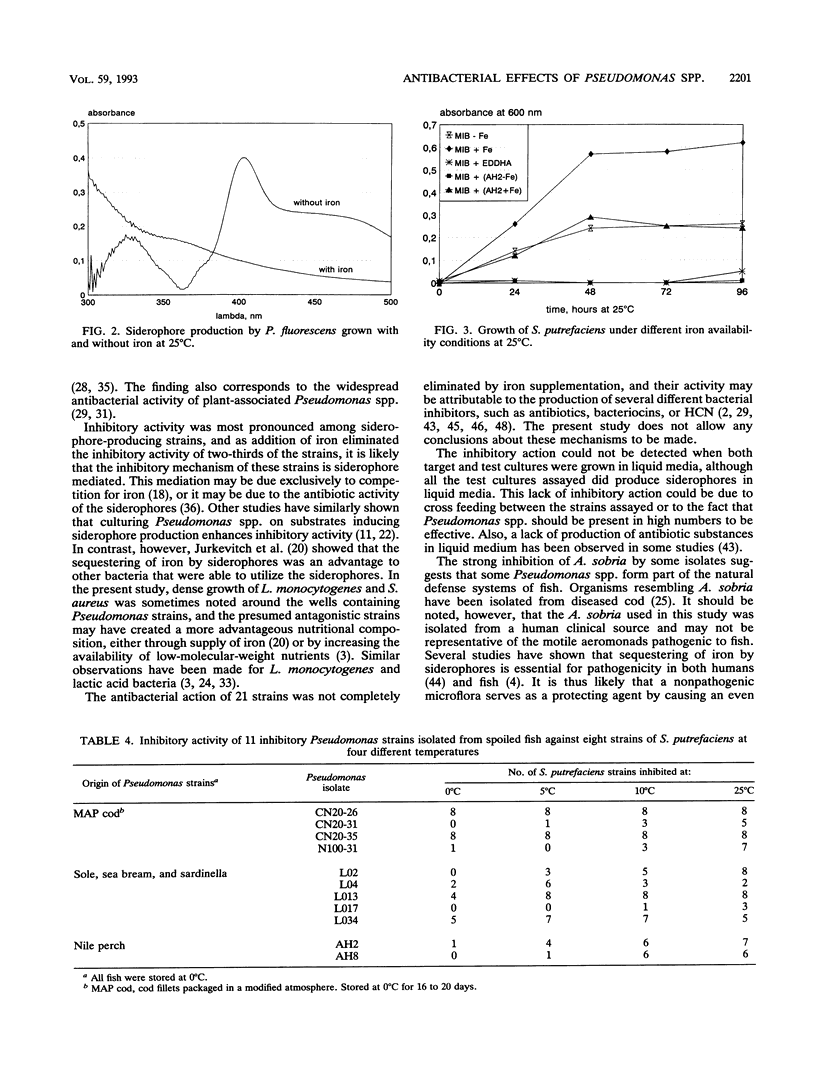
The antibacterial effects of 209 Pseudomonas strains isolated from spoiled iced fish and newly caught fish were assessed by screening target organisms in agar diffusion assays. One-third (67 strains) inhibited the growth of one or several of six target organisms (Escherichia coli, Shewanella putrefaciens, Aeromonas sobria, Pseudomonas fluorescens, Listeria monocytogenes, and Staphylococcus aureus), of which S. aureus and A. sobria were the most sensitive. The inhibitory action was most pronounced among the strains producing siderophores, and the presence of iron eliminated the antibacterial effect of two-thirds of the inhibitory strains. Siderophore-mediated competition for iron may explain the inhibitory activity of these strains. All but nine of the inhibiting strains were found to inhibit the growth of 38 psychrotrophic S. putrefaciens strains isolated from spoiling fish and fish products. Siderophore-containing Pseudomonas culture supernatants inhibited growth of S. putrefaciens, as did the addition of iron chelators (ethylenediamine dihydroxyphenylacetic acid [EDDHA]). In particular, Pseudomonas strains isolated from newly caught and spoiled Nile perch (Lates niloticus) inhibited S. putrefaciens. This suggests that microbial interaction (e.g., competition or antagonism) may influence the selection of a microflora for some chilled food products.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casida L. E. Competitive ability and survival in soil of pseudomonas strain 679-2, a dominant, nonobligate bacterial predator of bacteria. Appl Environ Microbiol. 1992 Jan;58(1):32–37. doi: 10.1128/aem.58.1.32-37.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. The relationship of plasmid-mediated iron transport and bacterial virulence. Annu Rev Microbiol. 1984;38:69–89. doi: 10.1146/annurev.mi.38.100184.000441. [DOI] [PubMed] [Google Scholar]
- Dopazo C. P., Lemos M. L., Lodeiros C., Bolinches J., Barja J. L., Toranzo A. E. Inhibitory activity of antibiotic-producing marine bacteria against fish pathogens. J Appl Bacteriol. 1988 Aug;65(2):97–101. doi: 10.1111/j.1365-2672.1988.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Edwards R. A., Dainty R. H., Hibbard C. M. Volatile compounds produced by meat pseudomonads and relate reference strains during growth on beef stored in air at chill temperatures. J Appl Bacteriol. 1987 May;62(5):403–412. doi: 10.1111/j.1365-2672.1987.tb02669.x. [DOI] [PubMed] [Google Scholar]
- FALKOW S. Activity of lysine decarboxlase as an aid in the identification of Salmonellae and Shigellae. Am J Clin Pathol. 1958 Jun;29(6):598–600. doi: 10.1093/ajcp/29.6_ts.598. [DOI] [PubMed] [Google Scholar]
- Fredrickson A. G., Stephanopoulos G. Microbial competition. Science. 1981 Aug 28;213(4511):972–979. doi: 10.1126/science.7268409. [DOI] [PubMed] [Google Scholar]
- Gennari M., Tomaselli S. Changes in aerobic microflora of skin and gills of Mediterranean sardines (Sardina pilchardus) during storage in ice. Int J Food Microbiol. 1988 Jun;6(4):341–347. doi: 10.1016/0168-1605(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Gram L., Wedell-Neergaard C., Huss H. H. The bacteriology of fresh and spoiling Lake Victorian Nile perch (Lates niloticus). Int J Food Microbiol. 1990 May;10(3-4):303–316. doi: 10.1016/0168-1605(90)90077-i. [DOI] [PubMed] [Google Scholar]
- HUGH R., LEIFSON E. The taxonomic significance of fermentative versus oxidative metabolism of carbohydrates by various gram negative bacteria. J Bacteriol. 1953 Jul;66(1):24–26. doi: 10.1128/jb.66.1.24-26.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan H., Weller D. M., Thomashow L. S. Relative importance of fluorescent siderophores and other factors in biological control of Gaeumannomyces graminis var. tritici by Pseudomonas fluorescens 2-79 and M4-80R. Appl Environ Microbiol. 1991 Nov;57(11):3270–3277. doi: 10.1128/aem.57.11.3270-3277.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurkevitch E., Hadar Y., Chen Y. Differential siderophore utilization and iron uptake by soil and rhizosphere bacteria. Appl Environ Microbiol. 1992 Jan;58(1):119–124. doi: 10.1128/aem.58.1.119-124.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Larsen J. L., Jensen N. J. An Aeromonas species implicated in ulcer-disease of the cod (Gadus morhua). Nord Vet Med. 1977 Apr-May;29(4-5):199–211. [PubMed] [Google Scholar]
- Leisinger T., Margraff R. Secondary metabolites of the fluorescent pseudomonads. Microbiol Rev. 1979 Sep;43(3):422–442. doi: 10.1128/mr.43.3.422-442.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren S. E., Dobrogosz W. J. Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiol Rev. 1990 Sep;7(1-2):149–163. doi: 10.1111/j.1574-6968.1990.tb04885.x. [DOI] [PubMed] [Google Scholar]
- Miller A., 3rd, Scanlan R. A., Lee J. S., Libbey L. M. Volatile compounds produced in sterile fish muscle (Sebastes melanops) by Pseudomonas putrefaciens, Pseudomonas fluorescens, and an Achromobacter species. Appl Microbiol. 1973 Jul;26(1):18–21. doi: 10.1128/am.26.1.18-21.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S., Simidu U. Distribution and significance of heterotrophic marine bacteria with antibacterial activity. Appl Environ Microbiol. 1987 Dec;53(12):2957–2962. doi: 10.1128/aem.53.12.2957-2962.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Olsson J. C., Westerdahl A., Conway P. L., Kjelleberg S. Intestinal colonization potential of turbot (Scophthalmus maximus)- and dab (Limanda limanda)-associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol. 1992 Feb;58(2):551–556. doi: 10.1128/aem.58.2.551-556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittard B. T., Freeman L. R., Later D. W., Lee M. L. Identification of volatile organic compounds produced by fluorescent pseudomonads on chicken breast muscle. Appl Environ Microbiol. 1982 Jun;43(6):1504–1506. doi: 10.1128/aem.43.6.1504-1506.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy M. C., Bills D. D., Lindsay R. C. Ester production by Pseudomonas fragi. II. Factors influencing ester levels in milk cultures. Appl Microbiol. 1969 Jun;17(6):779–782. doi: 10.1128/am.17.6.779-782.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B., Neilands J. B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987 Jan;160(1):47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Shanahan P., O'sullivan D. J., Simpson P., Glennon J. D., O'gara F. Isolation of 2,4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Appl Environ Microbiol. 1992 Jan;58(1):353–358. doi: 10.1128/aem.58.1.353-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand G. H., Anwar H., Kadurugamuwa J., Brown M. R., Silverman S. H., Melling J. In vivo evidence that bacteria in urinary tract infection grow under iron-restricted conditions. Infect Immun. 1985 Apr;48(1):35–39. doi: 10.1128/iai.48.1.35-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisard C., Keel C., Haas D., Dèfago G. Cyanide production by Pseudomonas fluorescens helps suppress black root rot of tobacco under gnotobiotic conditions. EMBO J. 1989 Feb;8(2):351–358. doi: 10.1002/j.1460-2075.1989.tb03384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerdahl A., Olsson J. C., Kjelleberg S., Conway P. L. Isolation and characterization of turbot (Scophtalmus maximus)-associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol. 1991 Aug;57(8):2223–2228. doi: 10.1128/aem.57.8.2223-2228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wratten S. J., Wolfe M. S., Andersen R. J., Faulkner D. J. Antibiotic metabolites from a marine pseudomonad. Antimicrob Agents Chemother. 1977 Mar;11(3):411–414. doi: 10.1128/aac.11.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]


