Abstract
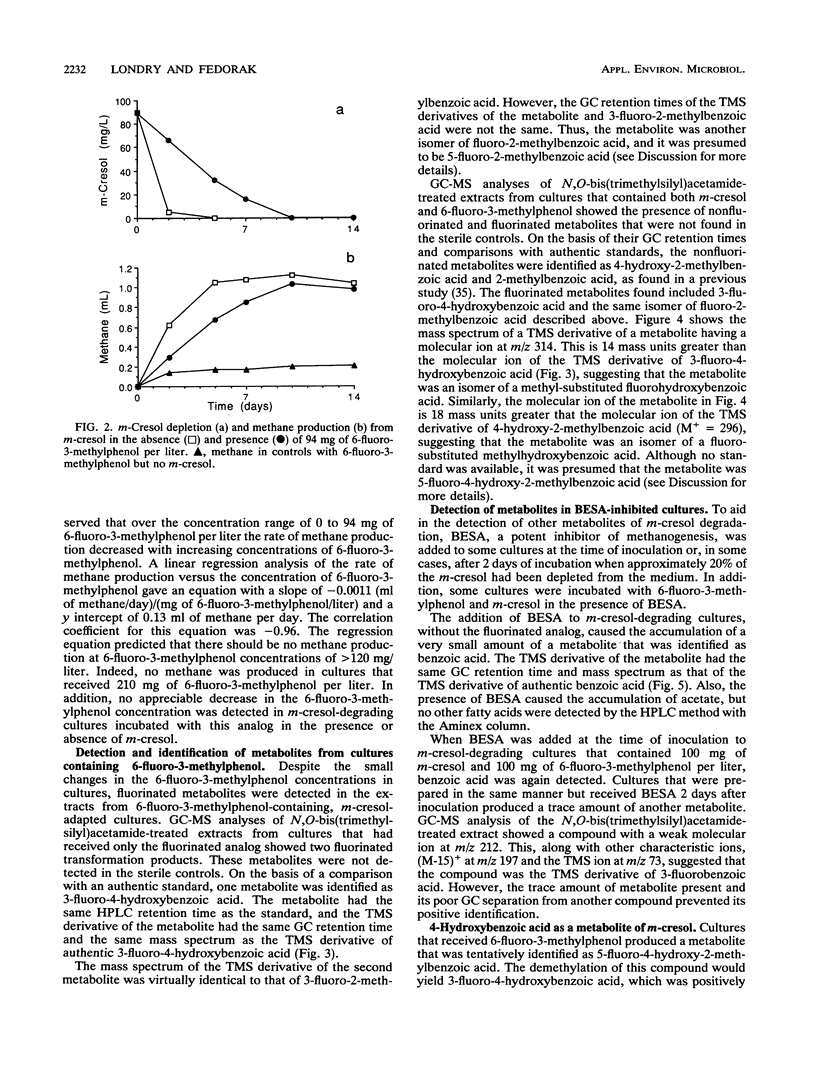
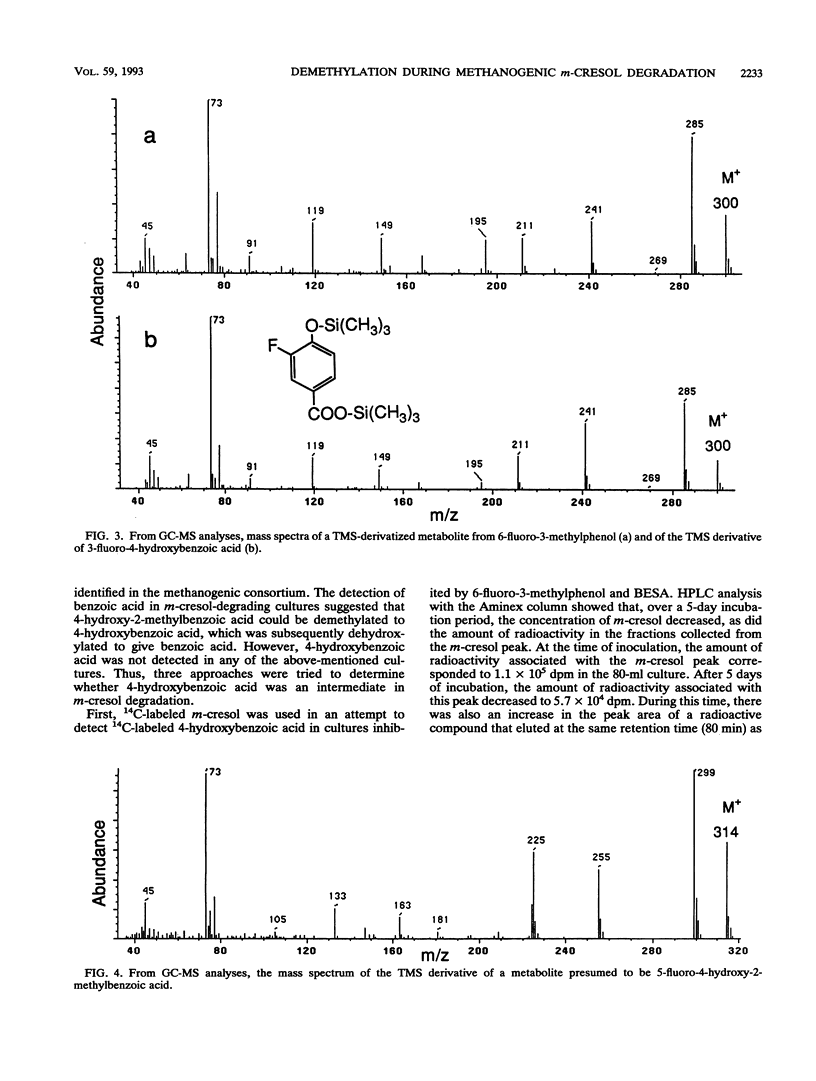
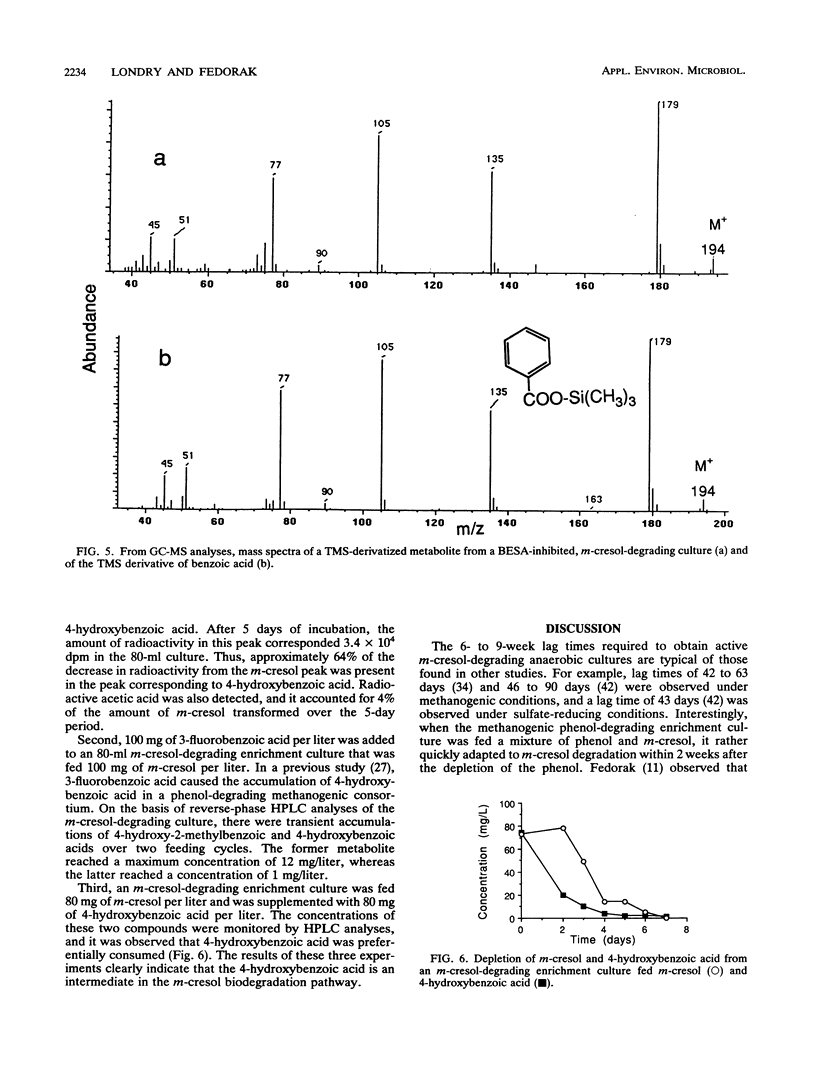
Anaerobic sewage sludge was used to enrich a methanogenic m-cresol-degrading consortium. 6-Fluoro-3-methylphenol was synthesized and added to subcultures of the consortium with m-cresol. This caused the accumulation of 4-hydroxy-2-methylbenzoic acid. In a separate experiment, the addition of 3-fluorobenzoic acid caused the transient accumulation of 4-hydroxybenzoic acid. Inhibition with bromoethanesulfonic acid caused the accumulation of benzoic acid. Thus, the proposed degradation pathway was m-cresol → 4-hydroxy-2-methylbenzoic acid → 4-hydroxybenzoic acid → benzoic acid. The m-cresol-degrading consortium was able to convert exogenous 4-hydroxybenzoic acid and benzoic acid to methane. In addition, for each metabolite of m-cresol identified, the corresponding fluorinated metabolite was detected, giving the following sequence: 6-fluoro-3-methylphenol → 5-fluoro-4-hydroxy-2-methylbenzoic acid → 3-fluoro-4-hydroxybenzoic acid → 3-fluorobenzoic acid. The second step in each of these pathways is a novel demethylation which was rate limiting. This demethylation reaction would likely facilitate the transformation of the methyl group to methane, which is consistent with the results of a previous study that showed that the methyl carbon of m-[methyl-14C]cresol was recovered predominantly as [14C]methane (D. J. Roberts, P. M. Fedorak, and S. E. Hrudey, Can. J. Microbiol. 33:335-338, 1987). The final aromatic compound in the proposed route for m-cresol metabolism was benzoic acid, and its detection in these cultures merges the pathway for the methanogenic degradation of m-cresol with those for the anaerobic metabolism of many phenols.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartle K. D., Elstub J., Novotny M., Robinson R. J. Use of a modified Tenax GC column packing for the direct gas chromatographic analysis of phenols in water at the ppm level. J Chromatogr. 1977 May 21;135(2):351–358. doi: 10.1016/s0021-9673(00)88375-2. [DOI] [PubMed] [Google Scholar]
- Bisaillon J. G., Lépine F., Beaudet R., Sylvestre M. Carboxylation of o-cresol by an anaerobic consortium under methanogenic conditions. Appl Environ Microbiol. 1991 Aug;57(8):2131–2134. doi: 10.1128/aem.57.8.2131-2134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd S. A., Shelton D. R., Berry D., Tiedje J. M. Anaerobic biodegradation of phenolic compounds in digested sludge. Appl Environ Microbiol. 1983 Jul;46(1):50–54. doi: 10.1128/aem.46.1.50-54.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudi F., Cardellini M., Cingolani G. M., Piergentili A., Peruzzi G., Balduini W. Synthesis and dopamine receptor affinities of 2-(4-fluoro-3- hydroxyphenyl)ethylamine and N-substituted derivatives. J Med Chem. 1990 Sep;33(9):2408–2412. doi: 10.1021/jm00171a014. [DOI] [PubMed] [Google Scholar]
- FINA L. R., FISKIN A. M. The anaerobic decomposition of benzoic acid during methane fermentation. II. Fate of carbons one and seven. Arch Biochem Biophys. 1960 Dec;91:163–165. doi: 10.1016/0003-9861(60)90483-5. [DOI] [PubMed] [Google Scholar]
- Frazer A. C., Young L. Y. Anaerobic c(1) metabolism of the o-methyl-C-labeled substituent of vanillate. Appl Environ Microbiol. 1986 Jan;51(1):84–87. doi: 10.1128/aem.51.1.84-87.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner B. R., Townsend G. T., Chapman P. J. para-hydroxybenzoate as an intermediate in the anaerobic transformation of phenol to benzoate. FEMS Microbiol Lett. 1991 Mar 1;62(2-3):265–269. doi: 10.1016/0378-1097(91)90168-a. [DOI] [PubMed] [Google Scholar]
- Goldman P. The carbon-fluorine bond in compounds of biological interest. Science. 1969 Jun 6;164(3884):1123–1130. doi: 10.1126/science.164.3884.1123. [DOI] [PubMed] [Google Scholar]
- Keith C. L., Bridges R. L., Fina L. R., Iverson K. L., Cloran J. A. The anaerobic decomposition of benzoic acid during methane fermentation. IV. Dearomatization of the ring and volatile fatty acids formed on ring rupture. Arch Microbiol. 1978 Aug 1;118(2):173–176. doi: 10.1007/BF00415726. [DOI] [PubMed] [Google Scholar]
- Londry K. L., Fedorak P. M. Benzoic acid intermediates in the anaerobic biodegradation of phenols. Can J Microbiol. 1992 Jan;38(1):1–11. doi: 10.1139/m92-001. [DOI] [PubMed] [Google Scholar]
- Lovley D. R., Lonergan D. J. Anaerobic Oxidation of Toluene, Phenol, and p-Cresol by the Dissimilatory Iron-Reducing Organism, GS-15. Appl Environ Microbiol. 1990 Jun;56(6):1858–1864. doi: 10.1128/aem.56.6.1858-1864.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanand K., Suflita J. M. Anaerobic degradation of m-cresol in anoxic aquifer slurries: carboxylation reactions in a sulfate-reducing bacterial enrichment. Appl Environ Microbiol. 1991 Jun;57(6):1689–1695. doi: 10.1128/aem.57.6.1689-1695.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. J., Fedorak P. M., Hrudey S. E. CO(2) Incorporation and 4-Hydroxy-2-Methylbenzoic Acid Formation during Anaerobic Metabolism of m-Cresol by a Methanogenic Consortium. Appl Environ Microbiol. 1990 Feb;56(2):472–478. doi: 10.1128/aem.56.2.472-478.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolphi A., Tschech A., Fuchs G. Anaerobic degradation of cresols by denitrifying bacteria. Arch Microbiol. 1991;155(3):238–248. doi: 10.1007/BF00252207. [DOI] [PubMed] [Google Scholar]
- Scheline R. R. Decarboxylation and demethylation of some phenolic benzoic acid derivatives by rat caecal contents. J Pharm Pharmacol. 1966 Oct;18(10):664–669. doi: 10.1111/j.2042-7158.1966.tb07780.x. [DOI] [PubMed] [Google Scholar]
- Smolenski W. J., Suflita J. M. Biodegradation of cresol isomers in anoxic aquifers. Appl Environ Microbiol. 1987 Apr;53(4):710–716. doi: 10.1128/aem.53.4.710-716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F. Aerobic and Anaerobic Catabolism of Vanillic Acid and Some Other Methoxy-Aromatic Compounds by Pseudomonas sp. Strain PN-1. Appl Environ Microbiol. 1983 Dec;46(6):1286–1292. doi: 10.1128/aem.46.6.1286-1292.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschech A., Fuchs G. Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch Microbiol. 1987 Sep;148(3):213–217. doi: 10.1007/BF00414814. [DOI] [PubMed] [Google Scholar]


