Abstract
The uidA gene, which encodes the beta-glucuronidase enzyme, was detected in 97.7% of 435 Escherichia coli isolates from treated and raw water sources by DNA-DNA hybridization; 92.4% of the strains expressed the translational product in 4-methylumbelliferyl-beta-D-glucuronide-containing media after reinoculation. Upon initial isolation from water samples, the minimal medium o-nitrophenyl-beta-D-galactopyranoside-4-methylum-belliferyl -beta-D-glucuronide preparations failed to detect more than 50% of the E. coli isolates that possessed uidA gene. Treated water gave the lowest recovery, with Colilert producing 26% positive samples and Coliquik producing 48% positive samples. There appears to be no relationship between the intensity of the autoradiographic signals of the uidA gene and the expression of beta-glucuronidase activity. Therefore, another variable such as physiological condition of the bacteria could be responsible for the nonexpression of the enzyme activity.
Full text
PDF

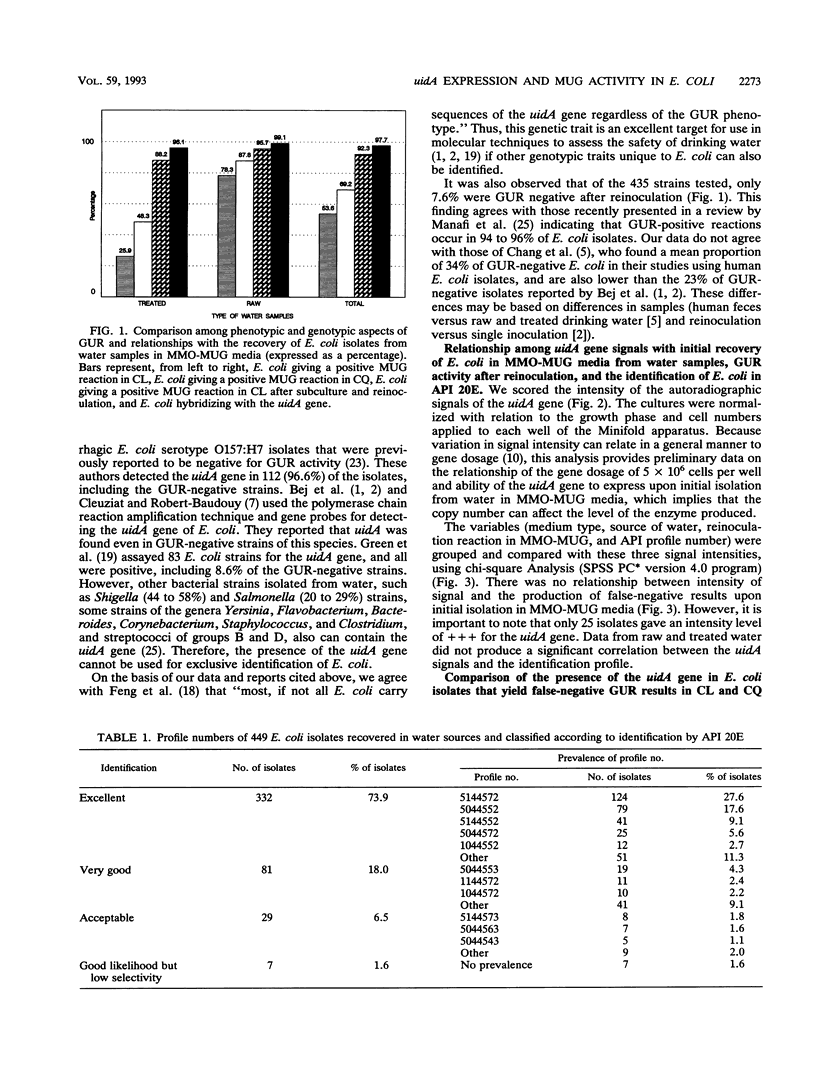
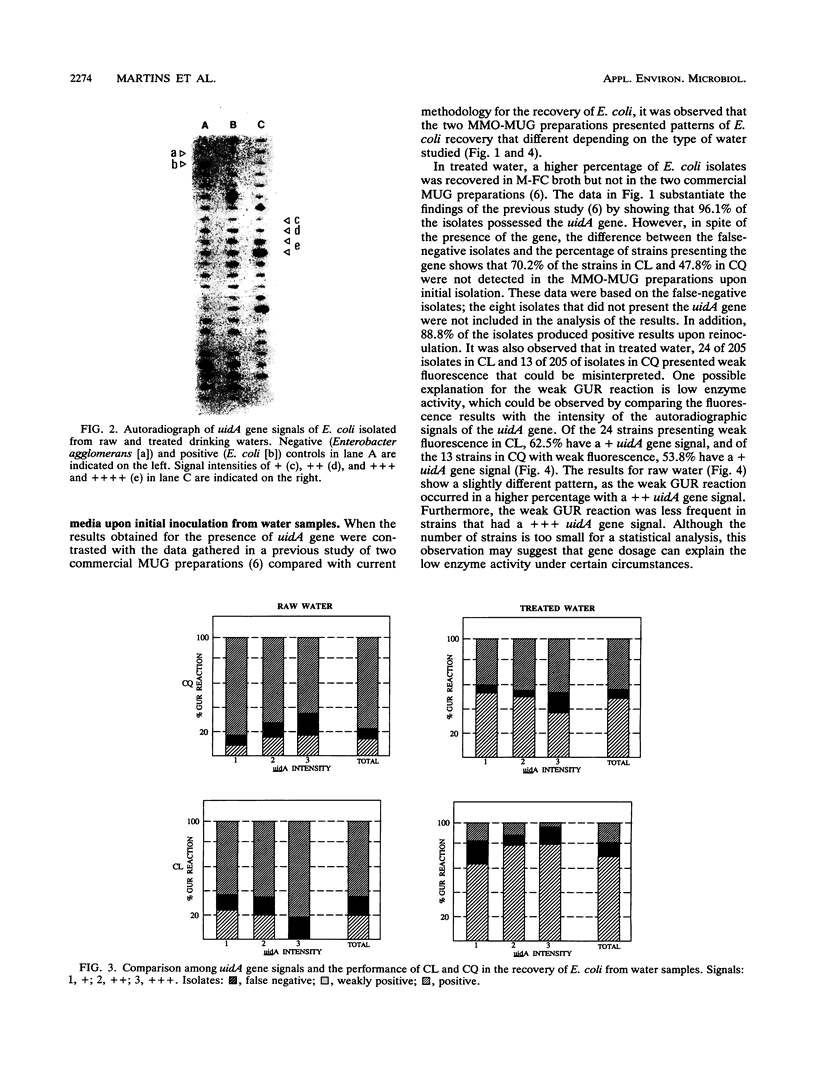
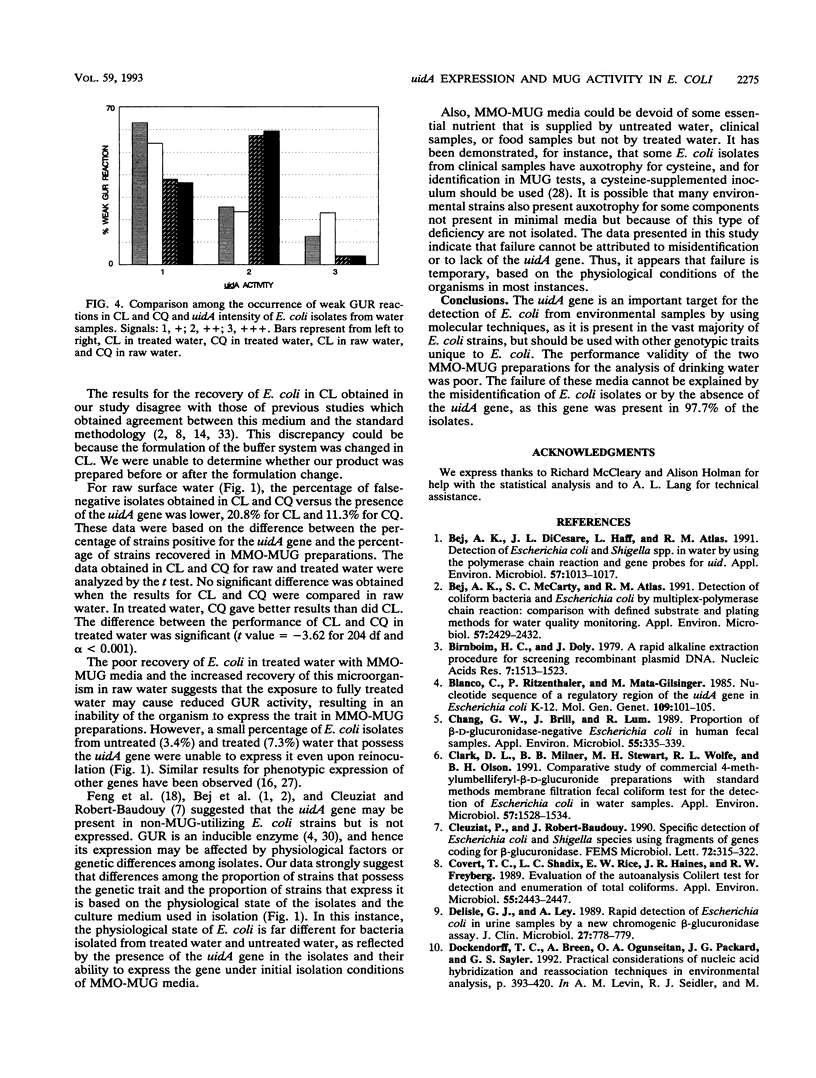

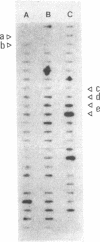
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bej A. K., DiCesare J. L., Haff L., Atlas R. M. Detection of Escherichia coli and Shigella spp. in water by using the polymerase chain reaction and gene probes for uid. Appl Environ Microbiol. 1991 Apr;57(4):1013–1017. doi: 10.1128/aem.57.4.1013-1017.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bej A. K., McCarty S. C., Atlas R. M. Detection of coliform bacteria and Escherichia coli by multiplex polymerase chain reaction: comparison with defined substrate and plating methods for water quality monitoring. Appl Environ Microbiol. 1991 Aug;57(8):2429–2432. doi: 10.1128/aem.57.8.2429-2432.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C., Ritzenthaler P., Mata-Gilsinger M. Nucleotide sequence of a regulatory region of the uidA gene in Escherichia coli K12. Mol Gen Genet. 1985;199(1):101–105. doi: 10.1007/BF00327517. [DOI] [PubMed] [Google Scholar]
- Chang G. W., Brill J., Lum R. Proportion of beta-D-glucuronidase-negative Escherichia coli in human fecal samples. Appl Environ Microbiol. 1989 Feb;55(2):335–339. doi: 10.1128/aem.55.2.335-339.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. L., Milner B. B., Stewart M. H., Wolfe R. L., Olson B. H. Comparative study of commercial 4-methylumbelliferyl-beta-D-glucuronide preparations with the Standard Methods membrane filtration fecal coliform test for the detection of Escherichia coli in water samples. Appl Environ Microbiol. 1991 May;57(5):1528–1534. doi: 10.1128/aem.57.5.1528-1534.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleuziat P., Robert-Baudouy J. Specific detection of Escherichia coli and Shigella species using fragments of genes coding for beta-glucuronidase. FEMS Microbiol Lett. 1990 Nov;60(3):315–322. doi: 10.1016/0378-1097(90)90324-j. [DOI] [PubMed] [Google Scholar]
- Covert T. C., Shadix L. C., Rice E. W., Haines J. R., Freyberg R. W. Evaluation of the Autoanalysis Colilert test for detection and enumeration of total coliforms. Appl Environ Microbiol. 1989 Oct;55(10):2443–2447. doi: 10.1128/aem.55.10.2443-2447.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisle G. J., Ley A. Rapid detection of Escherichia coli in urine samples by a new chromogenic beta-glucuronidase assay. J Clin Microbiol. 1989 Apr;27(4):778–779. doi: 10.1128/jcm.27.4.778-779.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour A. P., Strickland E. R., Cabelli V. J. Membrane filter method for enumerating Escherichia coli. Appl Environ Microbiol. 1981 May;41(5):1152–1158. doi: 10.1128/aem.41.5.1152-1158.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edberg S. C., Allen M. J., Smith D. B. National field evaluation of a defined substrate method for the simultaneous enumeration of total coliforms and Escherichia coli from drinking water: comparison with the standard multiple tube fermentation method. Appl Environ Microbiol. 1988 Jun;54(6):1595–1601. doi: 10.1128/aem.54.6.1595-1601.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T. M., Seidler R. J., LeChevallier M. W. Impact of verification media and resuscitation on accuracy of the membrane filter total coliform enumeration technique. Appl Environ Microbiol. 1981 May;41(5):1144–1151. doi: 10.1128/aem.41.5.1144-1151.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P. C., Hartman P. A. Fluorogenic assays for immediate confirmation of Escherichia coli. Appl Environ Microbiol. 1982 Jun;43(6):1320–1329. doi: 10.1128/aem.43.6.1320-1329.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P., Lum R., Chang G. W. Identification of uidA gene sequences in beta-D-glucuronidase-negative Escherichia coli. Appl Environ Microbiol. 1991 Jan;57(1):320–323. doi: 10.1128/aem.57.1.320-323.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Burgess S. M., Hirsh D. beta-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan C., Fitzgerald V. A., Dakin S. J., Behme R. J. Laboratory investigation of outbreak of hemorrhagic colitis caused by Escherichia coli O157:H7. J Clin Microbiol. 1987 Jun;25(6):1043–1047. doi: 10.1128/jcm.25.6.1043-1047.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manafi M., Kneifel W., Bascomb S. Fluorogenic and chromogenic substrates used in bacterial diagnostics. Microbiol Rev. 1991 Sep;55(3):335–348. doi: 10.1128/mr.55.3.335-348.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manafi M., Kneifel W. Ein kombiniertes Chromogen-Fluorogen-Medium zum simultanen Nachweis der Coliformengruppe und von E. coli in Wasser. Zentralbl Hyg Umweltmed. 1989 Dec;189(3):225–234. [PubMed] [Google Scholar]
- Mates A., Shaffer M. Membrane filtration differentiation of E. coli from coliforms in the examination of water. J Appl Bacteriol. 1989 Sep;67(3):343–346. doi: 10.1111/j.1365-2672.1989.tb02503.x. [DOI] [PubMed] [Google Scholar]
- McFeters G. A., Kippin J. S., LeChevallier M. W. Injured coliforms in drinking water. Appl Environ Microbiol. 1986 Jan;51(1):1–5. doi: 10.1128/aem.51.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIver C. J., Tapsall J. W. Assessment of conventional and commercial methods for identification of clinical isolates of cysteine-requiring strains of Escherichia coli and Klebsiella species. J Clin Microbiol. 1990 Sep;28(9):1947–1951. doi: 10.1128/jcm.28.9.1947-1951.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg L. J. Fluorogenic assay for rapid detection of Escherichia coli in food. Appl Environ Microbiol. 1985 Dec;50(6):1383–1387. doi: 10.1128/aem.50.6.1383-1387.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel M., Novel G. Regulation of beta-glucuronidase synthesis in Escherichia coli K-12: constitutive mutants specifically derepressed for uidA expression. J Bacteriol. 1976 Jul;127(1):406–417. doi: 10.1128/jb.127.1.406-417.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson B. H., Clark D. L., Milner B. B., Stewart M. H., Wolfe R. L. Total coliform detection in drinking water: comparison of membrane filtration with Colilert and Coliquik. Appl Environ Microbiol. 1991 May;57(5):1535–1539. doi: 10.1128/aem.57.5.1535-1539.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice E. W., Allen M. J., Brenner D. J., Edberg S. C. Assay for beta-glucuronidase in species of the genus Escherichia and its applications for drinking-water analysis. Appl Environ Microbiol. 1991 Feb;57(2):592–593. doi: 10.1128/aem.57.2.592-593.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice E. W., Allen M. J., Edberg S. C. Efficacy of beta-glucuronidase assay for identification of Escherichia coli by the defined-substrate technology. Appl Environ Microbiol. 1990 May;56(5):1203–1205. doi: 10.1128/aem.56.5.1203-1205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. B., Tomfohrde K. M., Rhoden D. L., Balows A. API system: a multitube micromethod for identification of Enterobacteriaceae. Appl Microbiol. 1972 Sep;24(3):449–452. doi: 10.1128/am.24.3.449-452.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y. L., Olson B. H. Rapid method for direct extraction of DNA from soil and sediments. Appl Environ Microbiol. 1991 Apr;57(4):1070–1074. doi: 10.1128/aem.57.4.1070-1074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Yu P. K., Martin W. J. Evaluation of accuracy of multitest micromethod system for identification of Enterobacteriaceae. Appl Microbiol. 1971 Sep;22(3):267–269. doi: 10.1128/am.22.3.267-269.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]