Abstract
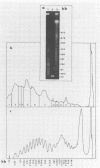
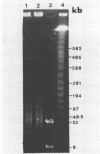
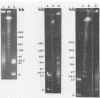
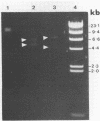
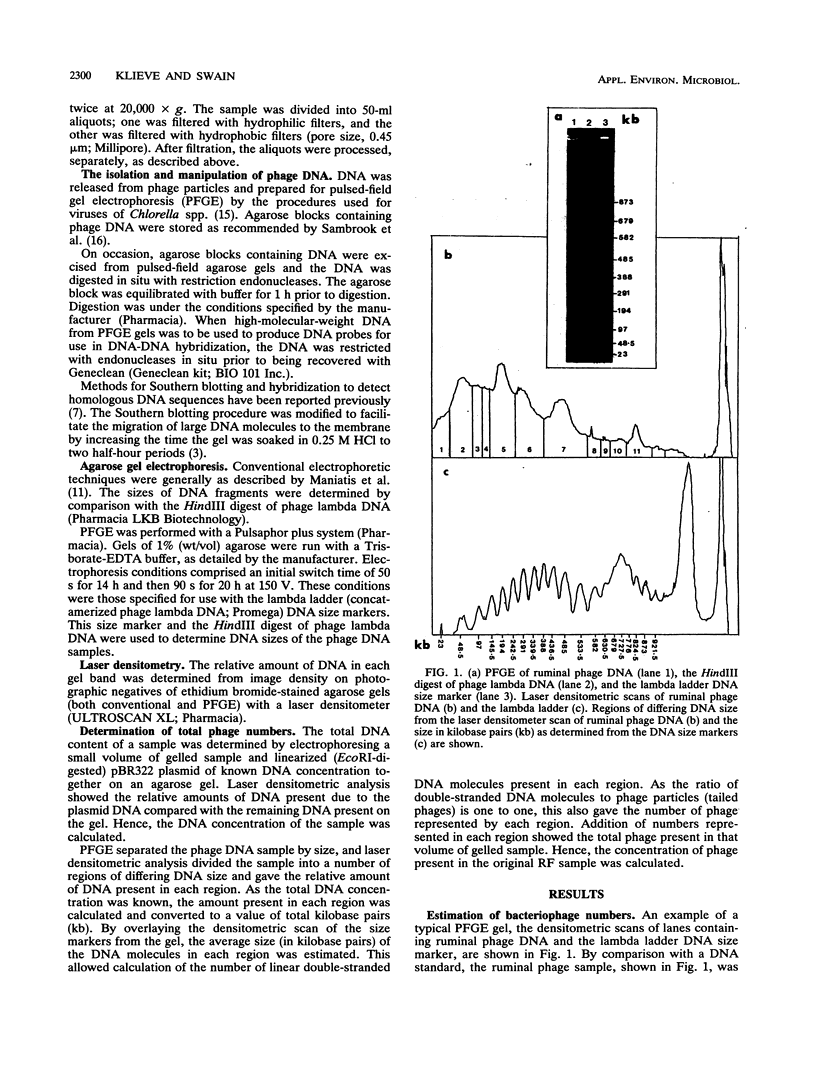
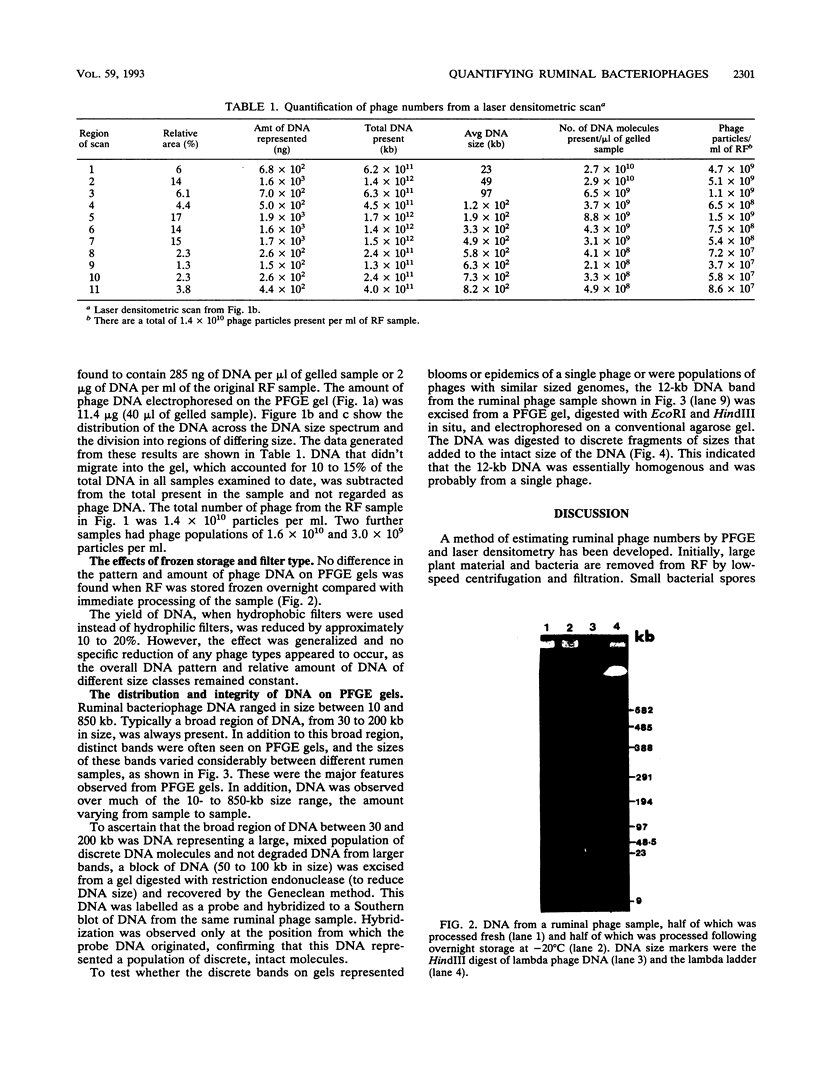
To investigate phage activity in the rumen, a method for quantifying phage has been developed. By differential centrifugation and ultrafiltration, phage particles were separated and concentrated from ruminal fluid. Linear double-stranded DNA from this fraction containing predominantly tailed phage was isolated and separated by size, using pulsed-field gel electrophoresis (PFGE). Laser densitometry of gel photographs allowed the numbers of phages with DNA in each size region to be calculated and, therefore, the total numbers per milliliter of ruminal fluid to be estimated. Phage numbers were estimated to be between 3 x 10(9) and 1.6 x 10(10) particles ml of ruminal fluid-1. The phage population, as gauged by the appearance of DNA on PFGE gels, had two major components. A broad region of DNA between 30 and 200 kb was always present on PFGE gels. It appears this region comprises DNA from a great many different phages and would include most of the temperate phages. In addition, discrete DNA bands ranging in size from 10 to 850 kb were frequently observed. DNA from one such band, of 12 kb in size, was shown to consist primarily of a single DNA type, suggesting that it originated from a specific phage. It is postulated that the discrete bands are due to epidemics or blooms of phage activity from specific, probably lytic, phages. The method that has been developed will greatly enhance future investigations into the interactions between the ruminal phage population, the ruminal bacterial population, and animal nutrition and growth.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernards A., Kooter J. M., Michels P. A., Moberts R. M., Borst P. Pulsed field gradient electrophoresis of DNA digested in agarose allows the sizing of the large duplication unit of a surface antigen gene in trypanosomes. Gene. 1986;42(3):313–322. doi: 10.1016/0378-1119(86)90235-0. [DOI] [PubMed] [Google Scholar]
- Klieve A. V., Bauchop T. Morphological diversity of ruminal bacteriophages from sheep and cattle. Appl Environ Microbiol. 1988 Jun;54(6):1637–1641. doi: 10.1128/aem.54.6.1637-1641.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klieve A. V., Bauchop T. Phage resistance and altered growth habit in a strain of Streptococcus bovis. FEMS Microbiol Lett. 1991 May 15;64(2-3):155–159. doi: 10.1016/0378-1097(91)90587-z. [DOI] [PubMed] [Google Scholar]
- Klieve A. V., Hudman J. F., Bauchop T. Inducible bacteriophages from ruminal bacteria. Appl Environ Microbiol. 1989 Jun;55(6):1630–1634. doi: 10.1128/aem.55.6.1630-1634.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockington R. A., Attwood G. T., Brooker J. D. Isolation and characterization of a temperate bacteriophage from the ruminal anaerobe Selenomonas ruminantium. Appl Environ Microbiol. 1988 Jun;54(6):1575–1580. doi: 10.1128/aem.54.6.1575-1580.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie R. I., White B. A. Recent advances in rumen microbial ecology and metabolism: potential impact on nutrient output. J Dairy Sci. 1990 Oct;73(10):2971–2995. doi: 10.3168/jds.S0022-0302(90)78986-2. [DOI] [PubMed] [Google Scholar]
- Nolan J. V., Leng R. A. Dynamic aspects of ammonia and urea metabolism in sheep. Br J Nutr. 1972 Jan;27(1):177–194. doi: 10.1079/bjn19720081. [DOI] [PubMed] [Google Scholar]
- Paynter M. J., Ewert D. L., Chalupa W. Some morphological types of bacteriophages in bovine rumen contents. Appl Microbiol. 1969 Nov;18(5):942–943. doi: 10.1128/am.18.5.942-943.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohozinski J., Girton L. E., Van Etten J. L. Chlorella viruses contain linear nonpermuted double-stranded DNA genomes with covalently closed hairpin ends. Virology. 1989 Feb;168(2):363–369. doi: 10.1016/0042-6822(89)90277-8. [DOI] [PubMed] [Google Scholar]
- Schwan W. R., Hartman P. A. A comparison of Selas and membrane filters for the sterilization of bacteriophage preparations. J Virol Methods. 1986 Sep;14(2):189–191. doi: 10.1016/0166-0934(86)90049-2. [DOI] [PubMed] [Google Scholar]
- Styriak I., Kmet V., Spanova A. Isolation and characterization of two rumen Streptococcus bovis bacteriophages. Microbiologica. 1989 Oct;12(4):317–322. [PubMed] [Google Scholar]