Abstract
Emerging evidence suggests that cocaine and other drugs of abuse can interfere with many aspects of cognitive functioning. The authors examined the effects of 0.1 – 15 mg/kg of cocaine on Pavlovian contextual and cued fear conditioning in mice. As expected, pre-training cocaine dose-dependently produced hyperactivity and disrupted freezing. Surprisingly, when the mice were tested off-drug later, the group pre-treated with a moderate dose of cocaine (15 mg/kg) displayed significantly less contextual and cued memory, compared to saline control animals. Conversely, mice pre-treated with a very low dose of cocaine (0.1 mg/kg) showed significantly enhanced fear memory for both context and tone, compared to controls. These results were not due to cocaine’s anesthetic effects, as shock reactivity was unaffected by cocaine. The data suggest that despite cocaine’s reputation as a performance-enhancing and anxiogenic drug, this effect is seen only at very low doses, whereas a moderate dose disrupts hippocampus and amygdala-dependent fear conditioning.
Keywords: Hippocampus, Amygdala, Freezing, Memory
A growing body of evidence supports the view that drugs of abuse interfere with many aspects of cognitive functioning. For example, cocaine use has been linked to deficits in such cognitive areas as attention, cognitive flexibility, and short-term and working memory [34, 43]. However, these findings in humans are controversial as cocaine use is naturally confounded with many other variables [e.g. 23, 27, 31]. Some studies, in fact, provide evidence for enhancement of certain cognitive abilities in cocaine users [e.g. 27, 50]. Relatively few studies in animals have examined the effects of cocaine on learning outside of the realm of addiction (e.g. self-administration, place preference, sensitization). Indeed, several studies have focused on the mechanisms underlying addiction, including structural plasticity [see 41 for review], but few have examined the acute, behavioral effects of cocaine in rodents on simple learning and memory paradigms. We believe that most researchers implicitly assume, as we once did, that cocaine, as a psychostimulant, would naturally enhance learning and memory. However, evidence now exists linking cocaine use with specific cognitive deficits [5, 25, 34, 43] as well as with general problems such as unemployment [35]. Moreover, a view has emerged that addictive drugs such as cocaine may work by taking control of critical reinforcement-related learning and memory circuits in the brain [28]. If cocaine modulates such circuits, then it may interfere with learning and memory. In this experiment, we examined the acute effects of cocaine on an aversively motivated Pavlovian fear conditioning task in mice.
In Pavlovian fear conditioning, an aversive, fear-producing unconditioned stimulus (US) is paired with an initially neutral conditioned stimulus (CS). The US elicits an unconditioned fear response (UR); following training, the CS alone elicits a conditioned fear response (CR). The environmental chamber where conditioning takes place may serve as a CS, a phenomenon known as context conditioning. A common fear conditioning paradigm is pairing a tone (CS) in a specific environmental context (CS) with a footshock (US). Although subjects manifest a number of physiological and behavioral changes after training, a commonly measured fear response is freezing [18]. Because Pavlovian fear conditioning can be monitored particularly efficiently in mice, it has become a popular means of assessing learning and memory in molecular studies [2].
The neuroanatomy of Pavlovian fear conditioning has been thoroughly investigated in recent years. The hippocampus is necessary for forming and temporarily storing memory of the context, while the amygdala is necessary for memory of both context and tone fear, and perhaps memory of the shock as well [1, 3, 19, 22, 38, 40]. Involvement of both the hippocampus [8, 36, 53, 54] and the amygdala [9, 16, 21, 37] has been implicated in cocaine use and addiction. For example, studies by Thompson, et al. [44, 45, 46] have shown that cocaine exposure modulates long-term potentiation in the CA1 region of the hippocampus. However, the cognitive and behavioral effects of acute cocaine administration on Pavlovian fear conditioning have yet to be examined.
In the current study, mice were injected with cocaine prior to placement in the conditioning chambers. Following training, subjects were tested for both context and tone memories, off drug. By one view, acute cocaine administration should potentiate fear conditioning, as cocaine is a nootropic psychostimulant [16] and an anxiogenic [4]. Studies have shown that cocaine can inhibit the extinction of a fear-potentiated startle response when paired repeatedly with a nonreinforced CS, suggesting that cocaine can intensify the anxiety-producing qualities of a CS [12]. Increased overall arousal or anxiety could enhance the learning of an anxiety-producing stimulus. Alternatively, cocaine could impair fear conditioning, as it disrupts not only the normal activation of the amygdala and hippocampus, but also areas in the prefrontal cortex related to attention [32]. According to this view, subjects on cocaine may not retain memory of context and tone cues because they would be unable to sufficiently attend to them during training. We were interested in characterizing the effects of cocaine on fear conditioning both with doses similar to those taken by human addicts, as well as lower doses, which we believed more likely to enhance learning. A moderate dose of cocaine produced a remarkable impairment in learning and memory, while a very low dose of cocaine enhanced memory. Several interpretations of these findings are presented.
Methods
Subjects
Sixty-four C57B6/J (B6; Jackson Laboratory, West Sacramento, CA) inbred mice were used. Mice were weaned at 3 weeks of age and were group housed (2–5 mice per cage) with continuous access to food and water. The vivarium was maintained on a 14:10 light/dark schedule, and all testing was performed during the light phase of the cycle. Mice were at least 8 weeks old before testing. Approximately equal numbers of male and female mice were used. All animal care and testing procedures were approved by the UCSD IACUC and were in accordance with the NIH Principles of Laboratory Animal Care.
Apparatus
Conditioning context
Three to four mice were tested concurrently, in individual conditioning chambers housed in a windowless room. Each chamber (32 cm wide, 25 cm high, 25 cm deep) was equipped with a speaker in the side wall and a stainless steel grid floor (36 rods, each rod 2-mm diameter, 8-mm center to center; the front wall was clear acrylic, the sidewalls were white acrylic; Med-Associates Inc., St. Albans, VT). A stainless steel drop-pan, scented with 7% isopropyl alcohol to provide a background odor, was located beneath each chamber. Between tests, the conditioning contexts were cleaned with 7% isopropyl alcohol solution. Background noise (65-dB) was provided by a HEPA air cleaner and white light was provided by two 100W bulbs. The mice were continually observed by a wall-mounted color video camera which was connected to a computer and video equipment in an adjacent room. Each chamber was connected to a solid-state scrambler, providing AC constant current shock, and an audio stimulus generator located in an adjacent room, controlled via an interface connected to a Windows computer running Med-PC (Med-Associates Inc., St Albans, VT). Automated assessment of freezing and activity was provided by custom designed software adapted from NIH Image running on an Apple Macintosh G4 [automated algorithm validated elsewhere, 2].
Alternate context
The alternate context for testing tone fear was located in a separate room that differed from the training context along several dimensions. Multiple (3–4) mice were tested concurrently, in individual boxes measuring 30 cm wide, 25 cm high, 24 cm deep, and equipped with a speaker in the side walls. The ceiling, floor, and three interior walls of the chamber were white, while a clear Plexiglas front wall allowed the mice to be continually observed. To create a distinct space, a white plastic, triangular tent was placed inside each box, with each side of the triangle measuring 23 cm. Between tests, the chambers were cleaned and scented with a 5% white vinegar solution. The room was lit with dim red light and an infrared video camera, connected to the Macintosh G4 described above, was used to score freezing.
Behavior Measurement
In order to measure the effect of cocaine on exploratory locomotor activity, baseline activity during training was assessed by counting the number of cross-overs each subject performed [e.g. 38]. A single cross-over was defined as the movement of a subject’s entire body from one half of the box to the other. Videotapes of the conditioning sessions were observed using a standard VCR and monitor, and the number of cross-overs were counted during the first 2 min prior to the first tone-shock pairing on the training day.
In addition, to ensure that cocaine did not disrupt shock reactivity, mouse activity burst displayed during the 2 s of shock exposure (Unconditioned Response to shock), as well as activity during the 2 s leading up to the shock were measured as velocity (cm/s) [2]. Full-screen video of this time period was digitized at 10 Hz using NIH Image. X-Y coordinates were obtained for each frame for each mouse using the wand auto-measure tool; these coordinates were imported into Microsoft Excel. Distance traveled (measured in pixels) between successive frames was computed using the distance formula [√((xn –xn+1)2 + (yn – yn+1)2)]; these values were converted into real distance in centimeters using known landmark distances in the video frame. Distance was then converted into velocity (cm/s) by dividing by time.
Drugs
Drugs were administered intraperitoneally (i.p.) in a volume of 5–10 ml/kg. Cocaine HCl (Sigma Chemical Co., St. Louis, MO) was dissolved in 0.9% physiological saline. Cocaine injections (salt weight; 15 mg/kg, 5 mg/kg, 1 mg/kg, or 0.1 mg/kg, i.p.) were given 15 min before training. These doses were selected to form a comprehensive dose-effect curve; although 15 mg/kg is usually regarded as a moderate dose, we found an unacceptable lethality rate (about one-third) at the next highest appropriate dose (30 mg/kg), in this strain of mice, whereas lower doses did not produce any lethality.
Experimental Procedures
Conditioning
Mice were injected with either saline or cocaine 15 min prior to training. Training consisted of a 2 min baseline period, followed by a 30 s tone, with a shock administered during the last 2 s of the tone. Two more tone-shock pairings followed, separated by 30 s each. Immediate post-shock freezing was measured for another 5 min. Thus, mice were inside the fear conditioning chambers for a total of 10 min, then returned to their home cages.
Testing
Mice were returned to the conditioning context without drug 24 h later and freezing was scored for 5 min. Mice were subsequently placed in the tone test context 48 h later. Tone testing consisted of a 2 min baseline, followed by a continuous 3 min tone identical to the training tone. Freezing was scored for the entire 5 min period.
Results
Generalized Activity
Cocaine produced an increase in cage cross-overs during the 2-min baseline period on the training day (Figure 1a). A univariate analysis of variance (ANOVA) confirmed group differences [F(4,59) = 7.1, p = 0.0001]. Compared to saline control animals, all doses of cocaine produced significant hyperactivity [0.1 mg/kg, F(1,24) = 12.2, p < 0.01; 1 mg/kg, F(1,24) = 12.5, p < 0.01; 5 mg/kg, F(1,24) = 14.0, p < 0.01; 15 mg/kg, F(1,26) = 17.0, p < 0.001], suggesting it is a psychomotor stimulant even at the lowest doses.
Figure 1.
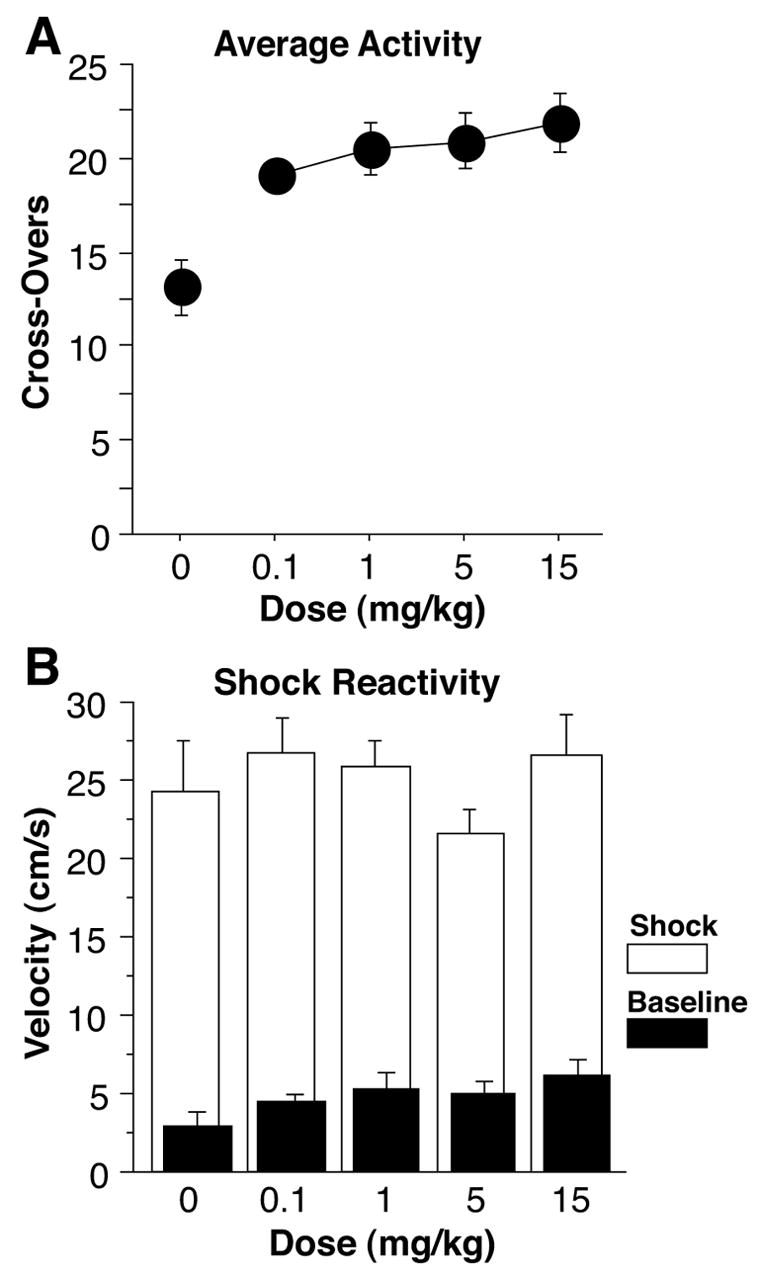
(a) Average Activity. Cage cross-overs were counted during the 2-min baseline period before the onset of the tone and shock on training day. Subjects receiving 0.1, 1, 5, or 15 mg/kg cocaine, i.p., 15-min prior to training showed hyperactivity compared to saline control subjects. (b) Shock Reactivity. Velocity was measured during the 2s prior to the first shock (Baseline) and during the 2s of the first shock (Shock). Only subjects receiving 15 mg/kg showed an increase in velocity during Baseline, compared to controls. All groups showed a marked increase in velocity from Baseline to Shock. The velocity during Shock did not differ between groups, showing no dose-dependence in Unconditioned Response.
Activity Burst Velocity
Cocaine produced an increase in baseline velocity in only the animals receiving 15 mg/kg in this very brief sample of velocity, compared to control animals [F(1,26) = 6.97, p < 0.05; all others, n.s.; Figure 1b]. Cocaine had no apparent effect on shock reactivity at any dose (data not shown), with no difference between doses in activity burst velocity during shock [F(4,59) = 0.8, p > 0.5]. The shock produced a large increase in velocity compared to baseline in all groups [F(4,59) = 428.0, p < 0.0001].
Training and Post-Shock Freezing
Figure 2a depicts the acquisition of freezing during training. The first 2 min period is a baseline measurement, and is followed by three tone-shock pairings spread over 3 min. Saline control subjects exhibited a higher level of freezing averaged over the 5 min training session compared with those receiving 1, 5, or 15 mg/kg, but did not differ from those receiving 0.1 mg/kg [0.1 mg/kg, F(1,24) = 2.92, p = 0.10; 1 mg/kg, F(1,24) = 5.57, p < 0.05; 5 mg/kg, F(1,24) = 25.0, p < 0.0001; 15 mg/kg, F(1,26) = 30.2, p < 0.0001]. Subjects receiving 5 or 15 mg/kg of cocaine displayed much less freezing than all other groups during the tone-shock pairings (F values > 25, p values < 0.0001). Figure 2b depicts the average level of freezing during an immediate memory test that consisted of leaving the mice in the chambers for an additional 5 min, on-drug. Dose-dependent effects on post-shock freezing are evident [ANOVA, F(4,59) = 22.1, p < 0.0001], with 0.1 mg/kg producing a nearly significant elevation in freezing, despite producing hyperactivity, compared with controls [F(1,24) = 4.1, p = 0.05]. There was no difference between saline and 1.0 mg/kg cocaine groups, while there was a large deficit in freezing in both the 5 mg/kg as well as 15 mg/kg, compared with saline controls (F values > 5, p values < 0.05). 15 mg/kg produced a larger deficit than 5 mg/kg [F(1,24) = 8.0, p < 0.01].
Figure 2.
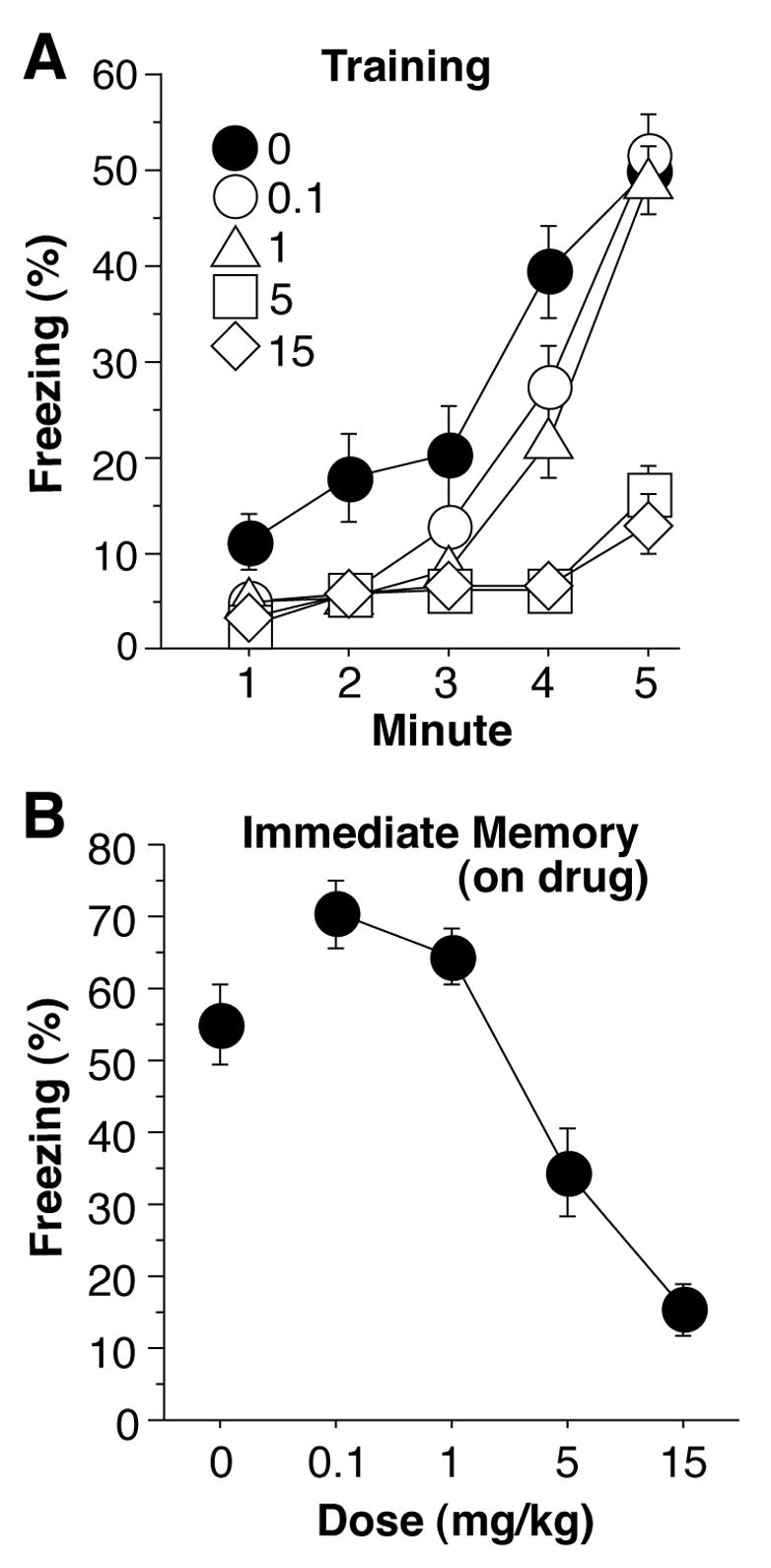
(a) Training. Freezing was measured continuously throughout the initial, 2-min baseline period, as well as the 3-min period encompassing 3 tone-shock pairings. Saline control subjects displayed more freezing than all other groups (0.1, 1, 5, and 15 mg/kg cocaine). Subjects receiving 5 or 15 mg/kg cocaine displayed much lower levels of freezing during the 3 tone-shock pairings. (b) Immediate Shock Memory. Freezing was measured for 5-min immediately following training, on drug. Dose-dependent effects are evident, with animals on 0.1 mg/kg cocaine displaying more freezing, than saline control animals. Animals administered with 5 or 15 mg/kg cocaine froze significantly less than controls.
This post-shock freezing measurement also, in part, addresses the issue of state-dependency in learning [e.g., 39]. The state-dependency view would predict that the decrement in freezing while on drug, during training, would be less than that seen off drug, during testing. However, the freezing decrement seen on drug is greater than that seen off drug.
Context Fear
One day after training, mice were returned to the context in which they had been trained, off-drug. A dose-dependent effect of cocaine can be seen in the average rate of freezing over the 5 min context test (Figure 3a) [ANOVA, F(4,59) = 7.8, p < 0.0001]. Subjects pretreated with 0.1 mg/kg during training displayed enhanced freezing, compared to saline controls, during the context test, off-drug [F(1,24) = 4.71, p < 0.05]. Mice pretreated with 15 mg/kg showed a significant decrement in freezing [F(1,26) = 8.88, p < 0.01], while those pretreated with 1 or 5 mg/kg were not significantly different than saline controls (F values < 1). In contrast to the immediate memory test, it is unlikely that response competition was a factor, since mice were tested off-drug, suggesting that cocaine given during training somehow interfered with the memory for fear conditioning. Another, although unlikely (see below) explanation is that conditioned hyperactivity, by virtue of drug-environment conditioning, produced an increase in activity in cocaine pre-treated mice that disrupted freezing.
Figure 3.
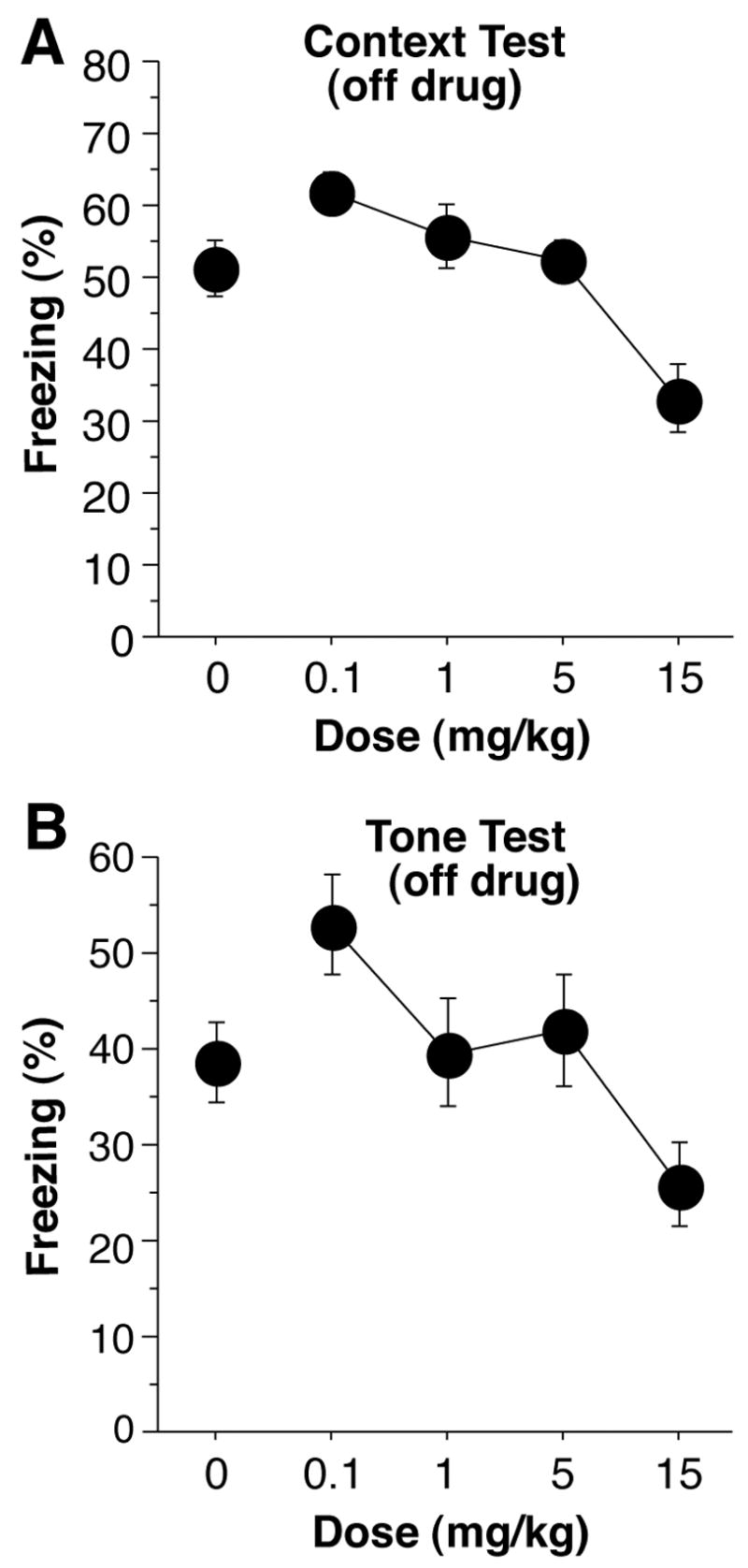
(a) Context Test. Twenty-four hours after conditioning, subjects were returned to the training context, off drug. Freezing was measured for 5-min. Subjects which had received 0.1 mg/kg of cocaine, pre-training, displayed an increase in freezing, compared to saline control (0) subjects. Subjects pre-treated with 15 mg/kg displayed a deficit in freezing. (b) Tone Test. Two days after training, subjects were introduced to a new context with no additional stimuli for 2-min, then presented with the tone from training for 3-min. Subjects given 0.1 mg/kg before training showed an increase in freezing, off drug, compared to saline controls, while those given 15 mg/kg showed a decrease in freezing.
Tone Fear
Two days after training, mice were administered the tone test in a novel context, off-drug. A dose-dependent response to the 3 min presentation of the tone is show in Figure 3b; there were significant differences [F(4,59) = 3.8, p < .01]. Mice administered 0.1 mg/kg cocaine, pre-training, displayed an increase in freezing during the tone test compared with saline controls [F(1,24) = 4.45, p < 0.05]. Mice pretreated with either 1 or 5 mg/kg displayed no difference in performance compared with controls [F values < 0.5], while mice administered 15 mg/kg showed a decrement in performance [F(1,26) = 4.50, p < 0.05]. These findings do not support the view that the cocaine pretreated mice exhibited deficits in freezing due to conditioned hyperactivity, since cocaine was never paired with this context. In addition, there was no difference in freezing between groups during the 2-min baseline period [F(4,59) = 0.9, p > 0.45; data not shown].
Overall, mice exhibited an enhancement of context and tone memory tested off drug when given 0.1 mg/kg, and a deficit in memory when given 15 mg/kg of cocaine. 5 mg/kg was also able to disrupt immediate memory, while the mice were still on the drug. Finally, doses of 0.1 to 15 mg/kg all produced hyperactivity, suggesting these are all still doses at which cocaine is acting as a psychostimulant.
Discussion
Administration of a very low dose of cocaine before fear conditioning enhanced freezing during both context and tone testing when subjects were off-drug, while a moderate dose disrupted memory during both tests. These results were surprising, as there are many reasons to expect a moderate dose of cocaine should potentiate fear conditioning. For example, cocaine is thought of as an anxiogenic, amplifying many anxiety and defensive behaviors in rodents [e.g. 4, 55], and thereby would be predicted to increase the response to fear conditioning. Cocaine has also been shown to increase the fear-potentiated startle response in rats, as well as inhibit extinction to a CS [10, 12, 52]. In addition, psychostimulants, such as caffeine, are usually thought of as nootropics [e.g. 15, 26, 49]. This folklore is exemplified by the advertisement campaign of the original Coca-Cola that contained cocaine, declaring it “the brain tonic and intellectual soda-fountain beverage” [51]. However, despite its anxiogenic and nootropic effects, only a very low dose of cocaine increased performance on fear conditioning, while a moderate dose disrupted it.
As fear conditioning is known to require activation of both the hippocampus and amygdala [1, 3, 19, 20, 38], one explanation for the results is that cocaine altered the regular functioning of the amygdala, the hippocampus, or both. In another study on fear conditioning, Corodimas, et al. [17] discovered the acute administration of caffeine, pre-training, produced a selective deficit in context conditioning only, suggesting a disruption of hippocampal function. Repeated exposure to cocaine can enhance long-term potentiation (LTP) in the CA1 region of the hippocampus [44]. However, this effect may be reversed at high concentrations of cocaine, or after long periods of withdrawal [45, 46], and has not been shown with an acute, in vivo exposure to cocaine. It is possible, though, that this enhanced LTP could account for the better performance during testing of the subjects which had received 0.1 mg/kg cocaine before training. Other studies on both acute and frequent cocaine intake have revealed the disruption of amygdala-dependent learning, as well [29, 48]. The present effects on both context and tone conditioning suggest that both hippocampus and amygdala-dependent learning can be disrupted even by acute, moderate cocaine administration, while a very low dose can enhance this learning.
One possibility is that cocaine interfered with motivational components of fear conditioning. Cocaine is a powerful local anesthetic, and it is possible that a 15 mg/kg, i.p. injection could have extended some anesthesia to the paws, reducing the painfulness of the footshock. However, measurement of the mouse activity burst UR suggests that footshock reactivity was equivalent in cocaine and saline-treated mice. Alternatively, the cocaine could have induced a pleasurable hedonic state, in which the animals on cocaine may have perceived the shock or fear conditioning experience as less aversive. This possibility would be difficult to explore within the current experiment, but could suggest that all drugs which induce a positive hedonic state may interfere with aversive conditioning. This seems unlikely, as there is evidence that cocaine, despite being thought of as producing a positive hedonic state, amplifies other anxiety and defensive behaviors [4]. Finally, there is also evidence from studies in monkeys that cocaine can disrupt an appetitively motivated working memory task as well [29].
Another possibility is that cocaine directly disrupted conditioning by interfering with learning and memory circuits. Human chronic cocaine users have been shown to have deficits in measures of attention [see 34, for a review], while dysfunction in prefrontal cortex activity has been implicated for attentional deficits in several studies of drug abusers [6, 7, 24, 25, 30, 32, 47]. Thus, it is possible that cocaine directly disrupted the circuits required for learning and memory in fear conditioning.
A possible confound in the present study is that we were unable to directly control for state-dependent learning. By this argument, on-drug testing may allow better recall of the memory of the context, tone and shock pairings, after on-drug training. Because the subjects were tested off-drug, one can argue that they were at a disadvantage, compared to controls, because of their different drug state during training and testing sessions. However, subjects which had received 0.1 mg/kg cocaine before training continued to show higher levels of freezing than controls when tested off-drug. State-dependent learning would predict quite the opposite result. Moreover, when the immediate memory test was used to control for state-dependency, mice were actually more sensitive to the disruptive effects of cocaine than when tested off-drug. These findings agree with previous research addressing similar issues. Studies examining the effects of repeated cocaine administration on fear conditioning, using the fear-potentiated startle paradigm, found that state-dependent learning did not satisfactorily account for increased startle amplitudes, after extinction [52]. Overall, there is little evidence that cocaine or other stimulants produce heavily state-dependent learning in humans or in other animals [39].
Although it is likely that hyperactivity directly attributable to the drug is responsible for freezing deficits seen during training, hyperactivity seems an unlikely explanation for deficits in context and tone memory when mice were tested off-drug. Conditioned hyperactivity via drug-environment conditioning could theoretically play a role in the apparent context and tone memory deficit seen in animals given 15 mg/kg cocaine. However, it is unlikely that much conditioned hyperactivity would be produced by a single drug exposure. Moreover, mice also exhibited deficits in tone testing, which was tested in a context never paired with cocaine, suggesting that conditioned hyperactivity played little role in the freezing deficits seen on testing. Finally, subjects which had been administered 0.1 mg/kg cocaine before training exhibited an increased level of activity during the 2 min baseline period, but exhibited increased freezing during both the context and tone tests, off-drug. Taken together this suggests that conditioned hyperactivity is not directly responsible for the memory deficits seen in context and tone conditioning.
An emerging view of addiction states that drugs of abuse hijack existing learning and memory systems normally involved in conditioned reinforcement [28]. If this is true, these neural systems may be unable to properly participate in the forms of memory they normally subserve. Given that cocaine alters the normal functioning of ventral tegmental area (VTA) – nucleus accumbens (NAcc) connectivity, it may disrupt the functional reward system of the brain. Human studies have implicated areas of the amygdala in this disruption, as well, correlating amygdala activation with cocaine craving [e.g. 13, 14]. If this is the case, then areas of the brain normally used for encoding the context and tone information during fear conditioning would be disrupted and unable to encode new information.
In addition, VTA dopamine neurons have been implicated in both the behavioral expression of a fear response as well as memory. For example, lesions to the VTA were shown to inhibit fear-potentiated startle in rats [11]. Also, cocaine administered repeatedly over 5–7 days, but not acutely on a single day, facilitated LTP induction in VTA dopamine neurons [33]. Together, these results suggest that repeated cocaine administration could enhance memory of a fear response via VTA dopamine neurons. These results also predict that an acute, moderate dose of cocaine, as used in the present study, would not be expected to enhance memory encoded via these neurons.
In the present study we chose to focus on a range of cocaine doses, from a low dose which is unlikely self-administered by drug users, to a moderate dose thought to be typical of drug users. Within that range, we found differing results. Future studies will use other psychostimulants [in preliminary studies with modafinil (Provigil®) we have found equivalent results]. We hope to determine whether a moderate dose of cocaine is unique in its detrimental effects on learning, or whether the acute administration of other stimulants may produce other previously unknown learning deficits, as well. Likewise, we will study if low doses of other psychostimulants also enhance learning.
These surprising results indicate that even an acute administration of a moderate dose of cocaine can be highly detrimental to learning and cognition. In particular, both hippocampus-dependent context memory and amygdala-dependent tone memory of Pavlovian conditioning were markedly disrupted by a moderate pre-training dose of cocaine. Taken together, these data suggest that many unexplored cognitive deficits may be produced by moderate doses of psychostimulant drugs that are normally thought of as performance-enhancing. Specifically, these renown performance-enhancing effects may only be seen at doses realistically too low to be administered by drug users.
Acknowledgments
SCW was supported by NSF Graduate Fellowship. These studies were supported by NIH (DA16635) and NARSAD awards to SGA. We thank Lindsay Godfrey for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anagnostaras SG, Dale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostaras SG, Josselyn SA, Frankland PW, Silva AJ. Computer-assisted behavioral assessment of Pavlovian fear conditioning in mice. Learn Mem. 2000;7:58–72. doi: 10.1101/lm.7.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanchard DC, Blanchard RJ. Cocaine potentiates defensive behaviors related to fear and anxiety. Neurosci Behav Rev. 1999;23:981–991. doi: 10.1016/s0149-7634(99)00031-7. [DOI] [PubMed] [Google Scholar]
- 5.Bolla KI, Cadet J, London ED. The neuropsychiatry of chronic cocaine abuse. J Neuropsychiat. 1998;10:280–289. doi: 10.1176/jnp.10.3.280. [DOI] [PubMed] [Google Scholar]
- 6.Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiat Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolouri MR, Small GA. Neuroimaging of hypoxia and cocaine-induced hippocampal stroke. J Neuroimaging. 2004;14:290–291. doi: 10.1177/1051228404265751. [DOI] [PubMed] [Google Scholar]
- 9.Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 10.Borowski TB, Kokkinidis L. The effects of cocaine, amphetamine, and the dopamine D1 receptor agonist SKF 38393 on fear extinction as measured with potentiated startle: implications for psychomotor stimulant psychosis. Behav Neurosci. 1998;112:952–965. doi: 10.1037//0735-7044.112.4.952. [DOI] [PubMed] [Google Scholar]
- 11.Borowski TB, Kokkinidis L. Contribution of ventral tegmental area dopamine neurons to expression of conditional fear: effects of electrical stimulation, excitotoxin lesions, and quinpirole infusion on potentiated startle in rats. Behav Neurosci. 1996;110:1349–1364. doi: 10.1037//0735-7044.110.6.1349. [DOI] [PubMed] [Google Scholar]
- 12.Borowski TB, Kokkinidis L. Cocaine preexposure sensitizes conditioned fear in a potentiated acoustic startle paradigm. Pharmacol Biochem Behav. 1994;49:935–942. doi: 10.1016/0091-3057(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 13.Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 14.Breiter HC, Rosen BR. Functional magnetic resonance imaging of brain reward circuitry in the human. Ann NY Acad Sci. 1999;877:523–547. doi: 10.1111/j.1749-6632.1999.tb09287.x. [DOI] [PubMed] [Google Scholar]
- 15.Brice CF, Smith AP. Effects of caffeine on mood and performance: a study of realistic consumption. Psychopharmacology. 2002;164:188–192. doi: 10.1007/s00213-002-1175-2. [DOI] [PubMed] [Google Scholar]
- 16.Cestari V, Mele A, Oliverio A, Castellano C. Amygdala lesions block the effect of cocaine on memory in mice. Brain Res. 1996;713:286–289. doi: 10.1016/0006-8993(95)01556-6. [DOI] [PubMed] [Google Scholar]
- 17.Corodimas KP, Pruitt JC, Stieg JM. Acute exposure to caffeine selectively disrupts context conditioning in rats. Psychopharmacology. 2000;152:376–382. doi: 10.1007/s002130000557. [DOI] [PubMed] [Google Scholar]
- 18.Fanselow MS. Conditional and unconditional components of post-shock freezing. Pav J Biol Sci. 1980;15:177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- 19.Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- 20.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 22.Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR, Wiltgen B, Fanselow MS. Role of the basolateral amygdala in the storage of fear memories across the adult lifetime in rats. J Neurosci. 2004;24:3810–3815. doi: 10.1523/JNEUROSCI.4100-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang G, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 25.Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- 26.Haskell CF, Kennedy DO, Wesnes KA, Scholey AB. Cognitive and mood improvements of caffeine in habitual consumers and habitual non-consumers of caffeine. Psychopharmacology. 2005;179:813–825. doi: 10.1007/s00213-004-2104-3. [DOI] [PubMed] [Google Scholar]
- 27.Hoff AL, Riordan H, Morris L, Cestaro V, Wieneke M, Alpert R, Wang G, Volkow N. Effects of crack cocaine on neurocognitive function. Psychiat Res. 1996;60:167–176. doi: 10.1016/0165-1781(96)02758-8. [DOI] [PubMed] [Google Scholar]
- 28.Hyman SE. Addiction: A disease of learning and memory. Am J Psychiat. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 29.Jentsch JD, Olausson P, De La Garza R, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology. 2002;26:183–190. doi: 10.1016/S0893-133X(01)00355-4. [DOI] [PubMed] [Google Scholar]
- 30.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 31.Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin and Exp Neuropsychology. 2005;27:189–204. doi: 10.1080/13803390490515694. [DOI] [PubMed] [Google Scholar]
- 32.Kantak KM, Udo T, Ugalde F, Luzzo C, Di Pietro N, Eichenbaum HB. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacology. 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- 33.Liu Q, Pu L, Poo M. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lundqvist T. Cognitive consequences of cannabis use: Comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacol Biochem Behav. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald Z, Pudney S. Illicit drug use, unemployment, and occupational attainment. J Health Economics. 2000;19:1089–1115. doi: 10.1016/s0167-6296(00)00056-4. [DOI] [PubMed] [Google Scholar]
- 36.Mackowiak M, Markowicz-Kula K, Fijal K, Wedzony K. Acute and repeated administration of cocaine differentially regulated expression of PSA-NCAM-positive neurons in the rat hippocampus. Brain Res. 2005;1055:149–155. doi: 10.1016/j.brainres.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Makris N, Gasic GP, Seidman LJ, Goldstein JM, Gastfriend DR, Elman I, Albaugh MD, Hodge SM, Ziegler DA, Sheahan FS, Caviness VS, Tsuang MT, Kennedy DN, Hyman SE, Rosen BR, Breiter HC. Decreased absolute amygdala volume in cocaine addicts. Neuron. 2004;44:729–740. doi: 10.1016/j.neuron.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 38.Maren S, Anagnostaras SG, Fanselow MS. The startled seahorse: is the hippocampus necessary for contextual fear conditioning? Trends Cog Sci. 1998;2:39–42. doi: 10.1016/s1364-6613(98)01123-1. [DOI] [PubMed] [Google Scholar]
- 39.Overton DA. State-dependent learning produced by addicting drugs. In: Fisher C, Freedman AM, editors. Opiate Addiction: Origins and Treatment. New York: Halsted Press Division of Wiley; 1972. pp. 61–75. [Google Scholar]
- 40.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 41.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 42.Rogerio R, Takahashi RN. Anxiogenic properties of cocaine in the rat evaluated with the elevated plus-maze. Pharmacol Biochem Behav. 1992;43:631–633. doi: 10.1016/0091-3057(92)90203-r. [DOI] [PubMed] [Google Scholar]
- 43.Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W. Cognitive performance of current methamphetamine and cocaine abusers. J Addictive Diseases. 2002;21:61–74. doi: 10.1300/j069v21n01_06. [DOI] [PubMed] [Google Scholar]
- 44.Thompson AM, Gosnell BA, Wagner JJ. Enhancement of long-term potentiation in the rat hippocampus following cocaine exposure. Neuropharmacology. 2002;42:1039–1042. doi: 10.1016/s0028-3908(02)00059-x. [DOI] [PubMed] [Google Scholar]
- 45.Thompson AM, Swant J, Gosnell BA, Wagner JJ. Modulation of long-term potentiation in the rat hippocampus following cocaine self-administration. Neuroscience. 2004;127:177–185. doi: 10.1016/j.neuroscience.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Thompson AM, Swant J, Wagner JJ. Cocaine-induced modulation of long-term potentiation in the CA1 region of rat hippocampus. Neuropharmacology. 2005;49:185–194. doi: 10.1016/j.neuropharm.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Trantham-Davidson H, Lavin A. Acute cocaine administration depresses cortical activity. Neuropsychopharmacology. 2004;29:2046–2051. doi: 10.1038/sj.npp.1300482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Udo T, Ugalde F, DiPietro N, Eichenbaum HB, Kantak KM. Effects of persistent cocaine self-administration on amygdala-dependent and dorsal striatum-dependent learning in rats. Psychopharmacology. 2004;174:237–245. doi: 10.1007/s00213-003-1734-1. [DOI] [PubMed] [Google Scholar]
- 49.van Duinen H, Lorist MM, Zijdewind I. The effect of caffeine on cognitive task performance and motor fatigue. Psychopharmacology. 2005;180:539–547. doi: 10.1007/s00213-005-2191-9. [DOI] [PubMed] [Google Scholar]
- 50.van Gorp WG, Wilkins JN, Hinkin CH, Moore LH, Hull J, Horner MD, Plotkin D. Declarative and procedural memory functioning in abstinent cocaine abusers. Arch Gen Psychiat. 1999;56:85–89. doi: 10.1001/archpsyc.56.1.85. [DOI] [PubMed] [Google Scholar]
- 51.Warburton DM. Addiction Controversies. Harwood Academic Publishers; Philadelphia: 1992. p. 23. [Google Scholar]
- 52.Willick ML, Kokkinidis L. Cocaine enhances the expression of fear-potentiated startle: evaluation of state-dependent extinction and the shock-sensitization of acoustic startle. Behav Neurosci. 1995;109:929–938. doi: 10.1037//0735-7044.109.5.929. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi M, Suzuki T, Seki T, Namba T, Juan R, Arai H, Hori T, Asada T. Repetitive cocaine administration decreases neurogenesis in adult rat hippocampus. Ann NY Acad Sci. 2004;1025:351–362. doi: 10.1196/annals.1316.043. [DOI] [PubMed] [Google Scholar]
- 54.Yamaguchi M, Suzuki T, Seki T, Namba T, Liu J, Arai H, Hori T, Shiga T. Decreased cell proliferation in the dentate gyrus of rats after repeated administration of cocaine. Synapse. 2005;58:63–71. doi: 10.1002/syn.20182. [DOI] [PubMed] [Google Scholar]
- 55.Yang X, Gorman AL, Dunn AJ, Goeders NE. Anxiogenic effects of acute and chronic cocaine administration: neurochemical and behavioral studies. Pharmacol Biochem Behav. 1992;41:643–650. doi: 10.1016/0091-3057(92)90386-t. [DOI] [PubMed] [Google Scholar]


