Abstract
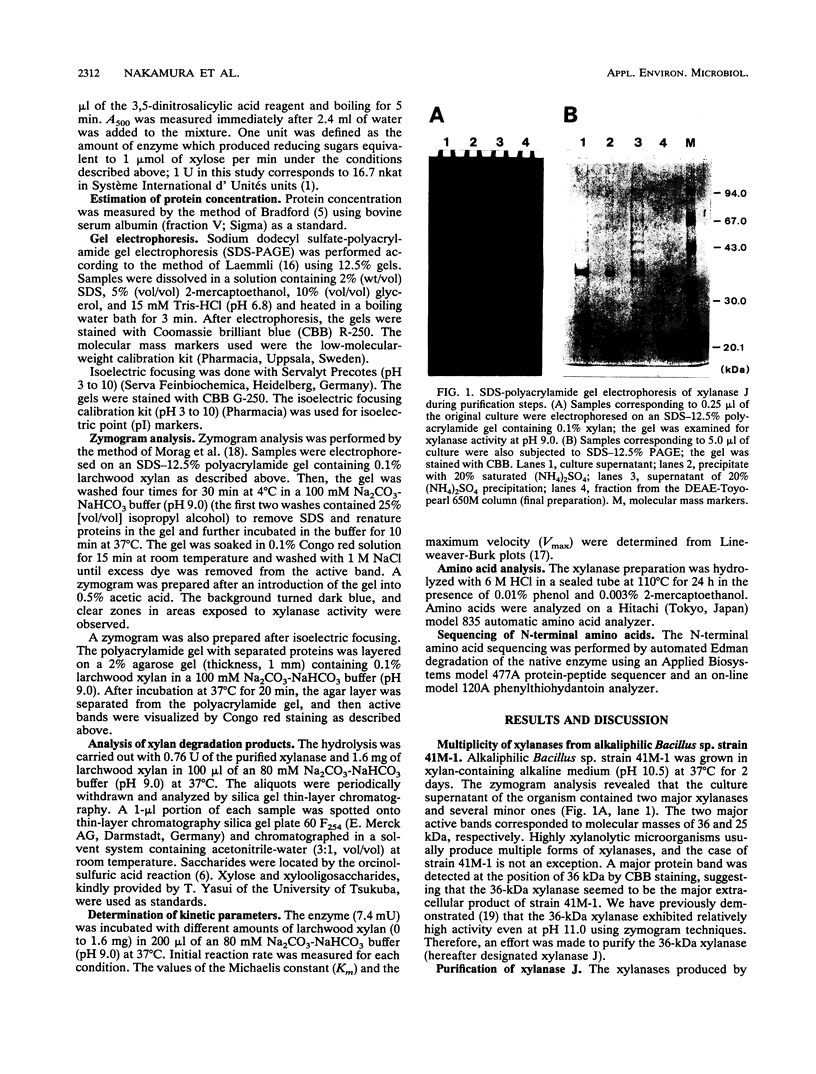
An alkaliphilic Bacillus sp. strain, 41M-1, isolated from soil produced multiple xylanases extracellularly. One of these xylanases was purified to homogeneity by ammonium sulfate fractionation and anion-exchange chromatography. The moleculr mass of this enzyme (xylanase J) was 36 kDa, and the isoelectric point was pH 5.3. Xylanase J was most active at pH 9.0. The optimum temperature for the activity at pH 9.0 was around 50 degrees C. The enzyme was stable up to 55 degrees C at pH 9.0 for 30 min. Xylanase J was completely inhibited by the Hg2+ion and N-bromosuccinimide. The predominant products of xylan hydrolysate were xylobiose, xylotriose, and higher oligosaccharides, indicating that the enzyme was an endoxylanase. The apparent Km and Vmax values on xylan were 3.3 mg/ml and 1,100 micromol-1 mg-1, respectively. Xylanase J showed high sequence homology with the xylanases from Bacillus pumilus and Clostridium acetobutylicum in the N-terminal region. Xylanase J acted on neither crystalline cellulose nor carboxymethyl cellulose, indicating a possible application of the enzyme in biobleaching processes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUCKNER J. Estimation of monosaccharides by the orcinol-sulphuric acid reaction. Biochem J. 1955 Jun;60(2):200–205. doi: 10.1042/bj0600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier R., Desrochers M., Jurasek L., Paice M. G. Isolation and Characterization of a Xylanase from Bacillus subtilis. Appl Environ Microbiol. 1983 Aug;46(2):511–514. doi: 10.1128/aem.46.2.511-514.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Deshpande V., Hinge J., Rao M. Chemical modification of xylanases: evidence for essential tryptophan and cysteine residues at the active site. Biochim Biophys Acta. 1990 Nov 15;1041(2):172–177. doi: 10.1016/0167-4838(90)90062-k. [DOI] [PubMed] [Google Scholar]
- Hrmová M., Petráková E., Biely P. Induction of cellulose- and xylan-degrading enzyme systems in Aspergillus terreus by homo- and heterodisaccharides composed of glucose and xylose. J Gen Microbiol. 1991 Mar;137(3):541–547. doi: 10.1099/00221287-137-3-541. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morag E., Bayer E. A., Lamed R. Relationship of cellulosomal and noncellulosomal xylanases of Clostridium thermocellum to cellulose-degrading enzymes. J Bacteriol. 1990 Oct;172(10):6098–6105. doi: 10.1128/jb.172.10.6098-6105.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer John C., Nakas J. P. Interrelationship of Xylanase Induction and Cellulase Induction of Trichoderma longibrachiatum. Appl Environ Microbiol. 1990 Aug;56(8):2535–2539. doi: 10.1128/aem.56.8.2535-2539.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. K., Tan L. U., Saddler J. N. Multiplicity of beta-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev. 1988 Sep;52(3):305–317. doi: 10.1128/mr.52.3.305-317.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappe H., Jones W. A., Woods D. R. Nucleotide sequence of a Clostridium acetobutylicum P262 xylanase gene (xynB). Nucleic Acids Res. 1990 Apr 25;18(8):2179–2179. doi: 10.1093/nar/18.8.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]






