Abstract
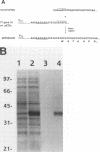
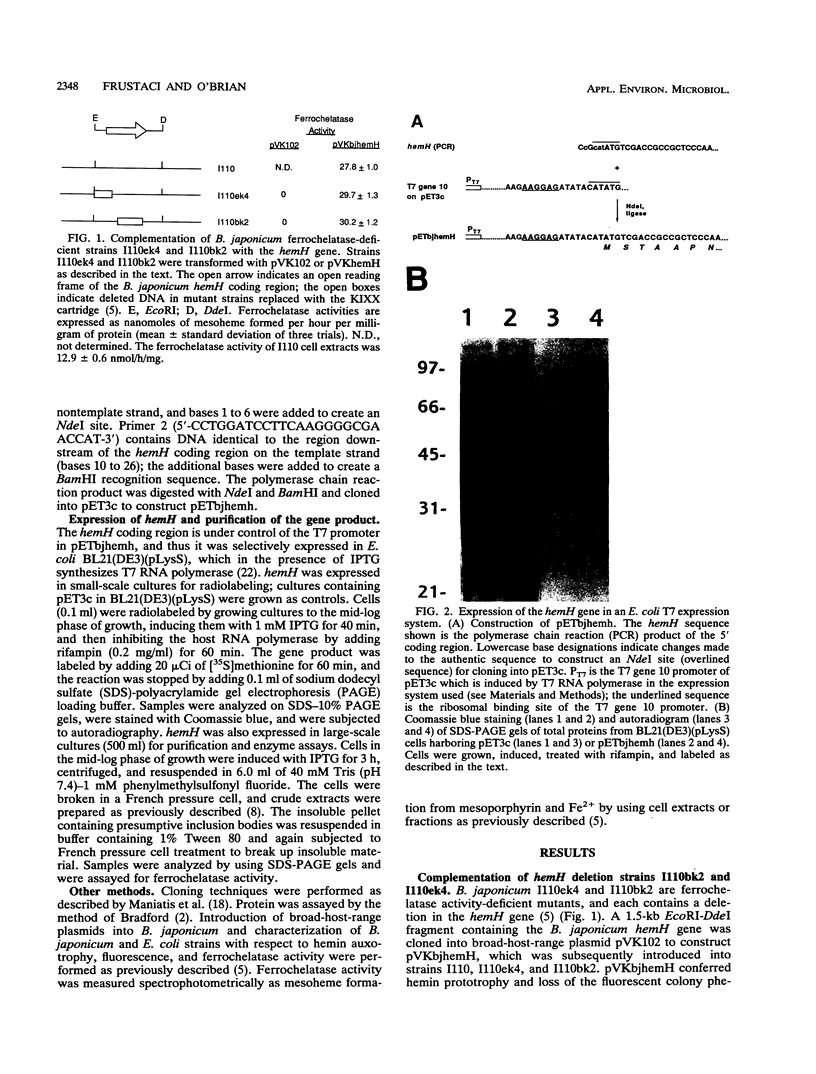
Complementation analysis showed that the Bradyrhizobium japonicum hemH gene was both necessary and sufficient to rescue mutant strains I110ek4 and I110bk2 in trans with respect to hemin auxotrophy, protoporphyrin accumulation, and the deficiency in ferrochelatase activity. The B. japonicum hemH gene was expressed in an Escherichia coli T7 expression system and yielded a 39-kDa protein, which was consistent with the predicted size of the deduced product. The overexpressed protein was purified and shown to contain ferrochelatase activity, thereby demonstrating that the hemH gene encodes ferrochelatase. When expressed from the lac promoter, the B. japonicum hemH gene was able to complement the enzyme activity of a ferrochelatase-defective E. coli mutant, and it also conferred hemin prototrophy on those cells. These latter findings confirm the identity of the hemH gene product and demonstrate that B. japonicum ferrochelatase can interact with the E. coli heme synthesis enzymes for heme formation in complemented cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., Frasier F. Cloning of murine ferrochelatase. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):849–853. doi: 10.1073/pnas.88.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira G. C., Andrew T. L., Karr S. W., Dailey H. A. Organization of the terminal two enzymes of the heme biosynthetic pathway. Orientation of protoporphyrinogen oxidase and evidence for a membrane complex. J Biol Chem. 1988 Mar 15;263(8):3835–3839. [PubMed] [Google Scholar]
- Frustaci J. M., O'Brian M. R. Characterization of a Bradyrhizobium japonicum ferrochelatase mutant and isolation of the hemH gene. J Bacteriol. 1992 Jul;174(13):4223–4229. doi: 10.1128/jb.174.13.4223-4229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci J. M., O'Brian M. R. The Escherichia coli visA gene encodes ferrochelatase, the final enzyme of the heme biosynthetic pathway. J Bacteriol. 1993 Apr;175(7):2154–2156. doi: 10.1128/jb.175.7.2154-2156.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frustaci J. M., Sangwan I., O'Brian M. R. Aerobic growth and respiration of a delta-aminolevulinic acid synthase (hemA) mutant of Bradyrhizobium japonicum. J Bacteriol. 1991 Feb;173(3):1145–1150. doi: 10.1128/jb.173.3.1145-1150.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhman I., Zamir A. The nucleotide sequence of the ferrochelatase and tRNA(val) gene region from Saccharomyces cerevisiae. Nucleic Acids Res. 1990 Oct 25;18(20):6130–6130. doi: 10.1093/nar/18.20.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot M. L., Chelm B. K. Bacterial delta-aminolevulinic acid synthase activity is not essential for leghemoglobin formation in the soybean/Bradyrhizobium japonicum symbiosis. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1837–1841. doi: 10.1073/pnas.83.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M., Hederstedt L. Cloning and characterization of the Bacillus subtilis hemEHY gene cluster, which encodes protoheme IX biosynthetic enzymes. J Bacteriol. 1992 Dec;174(24):8081–8093. doi: 10.1128/jb.174.24.8081-8093.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan P. M., Mgbeje B. I., Thomas S. D., Alwan A. F. Nucleotide sequence for the hemD gene of Escherichia coli encoding uroporphyrinogen III synthase and initial evidence for a hem operon. Biochem J. 1988 Jan 15;249(2):613–616. doi: 10.1042/bj2490613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Knauf V. C., Nester E. W. Wide host range cloning vectors: a cosmid clone bank of an Agrobacterium Ti plasmid. Plasmid. 1982 Jul;8(1):45–54. doi: 10.1016/0147-619x(82)90040-3. [DOI] [PubMed] [Google Scholar]
- Labbe-Bois R. The ferrochelatase from Saccharomyces cerevisiae. Sequence, disruption, and expression of its structural gene HEM15. J Biol Chem. 1990 May 5;265(13):7278–7283. [PubMed] [Google Scholar]
- Lamoril J., Boulechfar S., de Verneuil H., Grandchamp B., Nordmann Y., Deybach J. C. Human erythropoietic protoporphyria: two point mutations in the ferrochelatase gene. Biochem Biophys Res Commun. 1991 Dec 16;181(2):594–599. doi: 10.1016/0006-291x(91)91231-z. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Nakahigashi K., Nishimura K., Inokuchi H. Isolation and characterization of visible light-sensitive mutants of Escherichia coli K12. J Mol Biol. 1991 Jun 5;219(3):393–398. doi: 10.1016/0022-2836(91)90180-e. [DOI] [PubMed] [Google Scholar]
- Nakahashi Y., Taketani S., Okuda M., Inoue K., Tokunaga R. Molecular cloning and sequence analysis of cDNA encoding human ferrochelatase. Biochem Biophys Res Commun. 1990 Dec 14;173(2):748–755. doi: 10.1016/s0006-291x(05)80099-3. [DOI] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Molecular aspects of the energetics of nitrogen fixation in Rhizobium-legume symbioses. Biochim Biophys Acta. 1989 May 30;974(3):229–246. doi: 10.1016/s0005-2728(89)80239-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sangwan I., O'brian M. R. Characterization of delta-Aminolevulinic Acid Formation in Soybean Root Nodules. Plant Physiol. 1992 Mar;98(3):1074–1079. doi: 10.1104/pp.98.3.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangwan I., O'brian M. R. Evidence for an inter-organismic heme biosynthetic pathway in symbiotic soybean root nodules. Science. 1991 Mar 8;251(4998):1220–1222. doi: 10.1126/science.251.4998.1220. [DOI] [PubMed] [Google Scholar]
- Sasarman A., Nepveu A., Echelard Y., Dymetryszyn J., Drolet M., Goyer C. Molecular cloning and sequencing of the hemD gene of Escherichia coli K-12 and preliminary data on the Uro operon. J Bacteriol. 1987 Sep;169(9):4257–4262. doi: 10.1128/jb.169.9.4257-4262.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketani S., Nakahashi Y., Osumi T., Tokunaga R. Molecular cloning, sequencing, and expression of mouse ferrochelatase. J Biol Chem. 1990 Nov 15;265(32):19377–19380. [PubMed] [Google Scholar]