Abstract
The impact of promoter modification on the expression of the mosquitocidal Bacillus thuringiensis subsp. israelensis cryIVB gene when used to transform the cyanobacterium Synechococcus sp. strain PCC 7942 has been examined. Maximal transcript and protein abundances were achieved by the addition of the lacZ promoter upstream of the cryIVB sequence. Replacement of the endogenous corresponding Bacillus sequences with the Synechococcus petF1 promoter, ribosome binding site, and initiation codon also resulted in increased expression of the cryIVB gene relative to the expression obtained with the Bacillus promoter alone but decreased expression relative to the expression achieved with the tandem array of the Bacillus and lacZ promoters. Synechococcus cells carrying plasmids in which the expression of the cryIVB gene was regulated by either the lacZ or the petF1 promoter were readily consumed by first-instar Culex restuans larvae and proved to be toxic for these organisms.
Full text
PDF



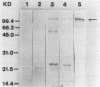
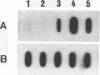
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angsuthanasombat C., Chungjatupornchai W., Kertbundit S., Luxananil P., Settasatian C., Wilairat P., Panyim S. Cloning and expression of 130-kd mosquito-larvicidal delta-endotoxin gene of Bacillus thuringiensis var. Israelensis in Escherichia coli. Mol Gen Genet. 1987 Jul;208(3):384–389. doi: 10.1007/BF00328128. [DOI] [PubMed] [Google Scholar]
- Angsuthanasombat C., Panyim S. Biosynthesis of 130-kilodalton mosquito larvicide in the cyanobacterium Agmenellum quadruplicatum PR-6. Appl Environ Microbiol. 1989 Sep;55(9):2428–2430. doi: 10.1128/aem.55.9.2428-2430.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly J., Coleman J. R. Effect of CO(2) Concentration on Protein Biosynthesis and Carbonic Anhydrase Expression in Chlamydomonas reinhardtii. Plant Physiol. 1988 Aug;87(4):833–840. doi: 10.1104/pp.87.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Expression of the Escherichia coli lacZ gene on a plasmid vector in a cyanobacterium. Science. 1985 Nov 15;230(4727):805–807. doi: 10.1126/science.2997920. [DOI] [PubMed] [Google Scholar]
- Delécluse A., Bourgouin C., Klier A., Rapoport G. Specificity of action on mosquito larvae of Bacillus thuringiensis israelensis toxins encoded by two different genes. Mol Gen Genet. 1988 Sep;214(1):42–47. doi: 10.1007/BF00340177. [DOI] [PubMed] [Google Scholar]
- Delécluse A., Charles J. F., Klier A., Rapoport G. Deletion by in vivo recombination shows that the 28-kilodalton cytolytic polypeptide from Bacillus thuringiensis subsp. israelensis is not essential for mosquitocidal activity. J Bacteriol. 1991 Jun;173(11):3374–3381. doi: 10.1128/jb.173.11.3374-3381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan W. P., Dankocsik C., Gilbert M. P. Molecular characterization of a gene encoding a 72-kilodalton mosquito-toxic crystal protein from Bacillus thuringiensis subsp. israelensis. J Bacteriol. 1988 Oct;170(10):4732–4738. doi: 10.1128/jb.170.10.4732-4738.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gendel S., Straus N., Pulleyblank D., Williams J. Shuttle cloning vectors for the cyanobacterium Anacystis nidulans. J Bacteriol. 1983 Oct;156(1):148–154. doi: 10.1128/jb.156.1.148-154.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- González J. M., Jr, Carlton B. C. A large transmissible plasmid is required for crystal toxin production in Bacillus thuringiensis variety israelensis. Plasmid. 1984 Jan;11(1):28–38. doi: 10.1016/0147-619x(84)90004-0. [DOI] [PubMed] [Google Scholar]
- Höfte H., Whiteley H. R. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev. 1989 Jun;53(2):242–255. doi: 10.1128/mr.53.2.242-255.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Murphy R. C., Stevens S. E., Jr Cloning and expression of the cryIVD gene of Bacillus thuringiensis subsp. israelensis in the cyanobacterium Agmenellum quadruplicatum PR-6 and its resulting larvicidal activity. Appl Environ Microbiol. 1992 May;58(5):1650–1655. doi: 10.1128/aem.58.5.1650-1655.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith M. E., Laudenbach D. E., Straus N. A. Isolation and nucleotide sequence analysis of the ferredoxin I gene from the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1986 Dec;168(3):1319–1324. doi: 10.1128/jb.168.3.1319-1324.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider G. J., Lang J. D., Haselkorn R. Promoter recognition by the RNA polymerase from vegetative cells of the cyanobacterium Anabaena 7120. Gene. 1991 Aug 30;105(1):51–60. doi: 10.1016/0378-1119(91)90513-b. [DOI] [PubMed] [Google Scholar]
- Thiery I., Nicolas L., Rippka R., Tandeau de Marsac N. Selection of cyanobacteria isolated from mosquito breeding sites as a potential food source for mosquito larvae. Appl Environ Microbiol. 1991 May;57(5):1354–1359. doi: 10.1128/aem.57.5.1354-1359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas W. E., Ellar D. J. Bacillus thuringiensis var israelensis crystal delta-endotoxin: effects on insect and mammalian cells in vitro and in vivo. J Cell Sci. 1983 Mar;60:181–197. doi: 10.1242/jcs.60.1.181. [DOI] [PubMed] [Google Scholar]
- Thorne L., Garduno F., Thompson T., Decker D., Zounes M., Wild M., Walfield A. M., Pollock T. J. Structural similarity between the lepidoptera- and diptera-specific insecticidal endotoxin genes of Bacillus thuringiensis subsp. "kurstaki" and "israelensis". J Bacteriol. 1986 Jun;166(3):801–811. doi: 10.1128/jb.166.3.801-811.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitukaitis J. L. Production of antisera with small doses of immunogen: multiple intradermal injections. Methods Enzymol. 1981;73(Pt B):46–52. doi: 10.1016/0076-6879(81)73055-6. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., Dullemans A. M., van Workum M. E., Visser B. Molecular cloning and the nucleotide sequence of the Mr 28 000 crystal protein gene of Bacillus thuringiensis subsp. israelensis. Nucleic Acids Res. 1985 Nov 25;13(22):8207–8217. doi: 10.1093/nar/13.22.8207. [DOI] [PMC free article] [PubMed] [Google Scholar]