Abstract
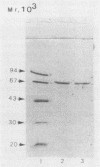
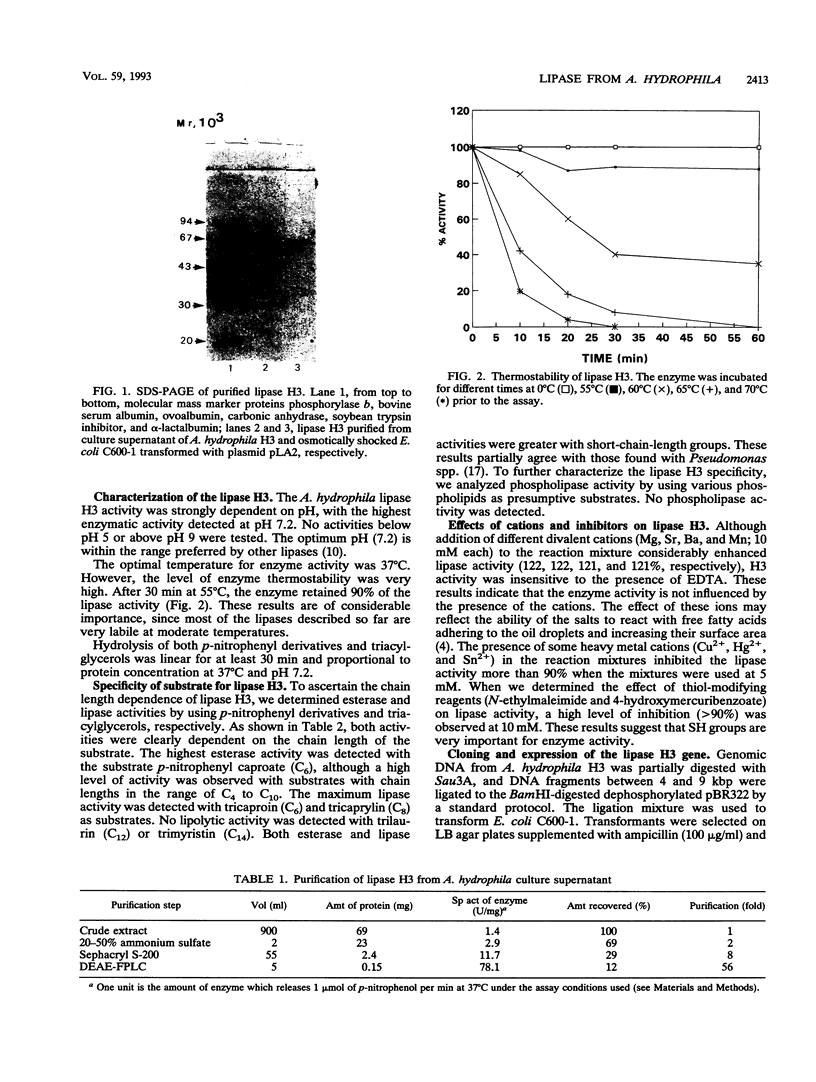
A structural gene which codes for an extracellular lipase (EC 3.1.1.3) in Aeromonas hydrophila H3, which was isolated from a female hospitalized patient, was cloned in Escherichia coli by using pBR322 as a vector. Lipase purified from both A. hydrophila culture supernatant and the periplasmic fluids of E. coli containing the lip determinant in the original clone (plasmid pLA2) showed an M(r) of 67,000 on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, which agrees with the M(r) determined by Sephacryl S-200 chromatography. Regarding substrate specificity, the optimum chain lengths for the acyl moiety were C6 for ester hydrolysis and C6 and C8 for triacylglycerol hydrolysis. Sequence analysis showed a major open reading frame of 2,052 bp, which predicts a polypeptide with an M(r) of 71,804. The polypeptide was found to contain an amino acid sequence (V-H-F-L-G-H-S-L-G-A) which is highly preserved among lipases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anguita J., Rivero O., Naharro G. Molecular Cloning of the Tryptophan Operon from an Aeromonas hydrophila Freshwater Isolate. Appl Environ Microbiol. 1992 Mar;58(3):1031–1034. doi: 10.1128/aem.58.3.1031-1034.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Buttke T. M., Cuchens M. A. Inhibition of lymphocyte proliferation by free fatty acids. II. Toxicity of stearic acid towards phytohaemagglutinin-activated T cells. Immunology. 1984 Nov;53(3):507–514. [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T., Huhle B., Bergbauer H., Goebel W. Cloning, expression, and mapping of the Aeromonas hydrophila aerolysin gene determinant in Escherichia coli K-12. J Bacteriol. 1986 Jul;167(1):368–374. doi: 10.1128/jb.167.1.368-374.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T., Huhle B., Hof H., Bergbauer H., Goebel W. Marker exchange mutagenesis of the aerolysin determinant in Aeromonas hydrophila demonstrates the role of aerolysin in A. hydrophila-associated systemic infections. Infect Immun. 1987 Sep;55(9):2274–2280. doi: 10.1128/iai.55.9.2274-2280.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derewenda Z. S., Sharp A. M. News from the interface: the molecular structures of triacylglyceride lipases. Trends Biochem Sci. 1993 Jan;18(1):20–25. doi: 10.1016/0968-0004(93)90082-x. [DOI] [PubMed] [Google Scholar]
- Eftimiadi C., Buzzi E., Tonetti M., Buffa P., Buffa D., van Steenbergen M. T., de Graaff J., Botta G. A. Short-chain fatty acids produced by anaerobic bacteria alter the physiological responses of human neutrophils to chemotactic peptide. J Infect. 1987 Jan;14(1):43–53. doi: 10.1016/s0163-4453(87)90808-5. [DOI] [PubMed] [Google Scholar]
- Gilbert E. J., Cornish A., Jones C. W. Purification and properties of extracellular lipase from Pseudomonas aeruginosa EF2. J Gen Microbiol. 1991 Sep;137(9):2223–2229. doi: 10.1099/00221287-137-9-2223. [DOI] [PubMed] [Google Scholar]
- Harwood J. The versatility of lipases for industrial uses. Trends Biochem Sci. 1989 Apr;14(4):125–126. doi: 10.1016/0968-0004(89)90140-0. [DOI] [PubMed] [Google Scholar]
- Hawley H. P., Gordon G. B. The effects of long chain free fatty acids on human neutrophil function and structure. Lab Invest. 1976 Feb;34(2):216–222. [PubMed] [Google Scholar]
- Hilton S., Buckley J. T. Studies on the reaction mechanism of a microbial lipase/acyltransferase using chemical modification and site-directed mutagenesis. J Biol Chem. 1991 Jan 15;266(2):997–1000. [PubMed] [Google Scholar]
- Hilton S., McCubbin W. D., Kay C. M., Buckley J. T. Purification and spectral study of a microbial fatty acyltransferase: activation by limited proteolysis. Biochemistry. 1990 Sep 25;29(38):9072–9078. doi: 10.1021/bi00490a026. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Ihara F., Kageyama Y., Hirata M., Nihira T., Yamada Y. Purification, characterization, and molecular cloning of lactonizing lipase from Pseudomonas species. J Biol Chem. 1991 Sep 25;266(27):18135–18140. [PubMed] [Google Scholar]
- Jauhiainen M., Dolphin P. J. Human plasma lecithin-cholesterol acyltransferase. An elucidation of the catalytic mechanism. J Biol Chem. 1986 May 25;261(15):7032–7043. [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leung K. Y., Stevenson R. M. Tn5-induced protease-deficient strains of Aeromonas hydrophila with reduced virulence for fish. Infect Immun. 1988 Oct;56(10):2639–2644. doi: 10.1128/iai.56.10.2639-2644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahari K., Tanaka T., Hishinuma F., Kuroda M., Sakaguchi K. Control of tryptophan synthetase amplified by varying the numbers of composite plasmids in Escherichia coli cells. Gene. 1977 Mar;1(2):141–152. doi: 10.1016/0378-1119(77)90025-7. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Nieto T. P., Santos Y., Rodriguez L. A., Ellis A. E. An extracellular acetylcholinesterase produced by Aeromonas hydrophila is a major lethal toxin for fish. Microb Pathog. 1991 Aug;11(2):101–110. doi: 10.1016/0882-4010(91)90003-s. [DOI] [PubMed] [Google Scholar]
- Paniagua C., Rivero O., Anguita J., Naharro G. Pathogenicity factors and virulence for rainbow trout (Salmo gairdneri) of motile Aeromonas spp. isolated from a river. J Clin Microbiol. 1990 Feb;28(2):350–355. doi: 10.1128/jcm.28.2.350-355.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero O., Anguita J., Mateos D., Paniagua C., Naharro G. Cloning and characterization of an extracellular temperature-labile serine protease gene from Aeromonas hydrophila. FEMS Microbiol Lett. 1991 Jun 1;65(1):1–7. doi: 10.1016/0378-1097(91)90461-i. [DOI] [PubMed] [Google Scholar]
- Rivero O., Anguita J., Paniagua C., Naharro G. Molecular cloning and characterization of an extracellular protease gene from Aeromonas hydrophila. J Bacteriol. 1990 Jul;172(7):3905–3908. doi: 10.1128/jb.172.7.3905-3908.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. L., Hilton S., Buckley J. T. Stereochemical and positional specificity of the lipase/acyltransferase produced by Aeromonas hydrophila. Biochemistry. 1992 Jun 2;31(21):4974–4980. doi: 10.1021/bi00136a009. [DOI] [PubMed] [Google Scholar]
- Rollof J., Braconier J. H., Söderström C., Nilsson-Ehle P. Interference of Staphylococcus aureus lipase with human granulocyte function. Eur J Clin Microbiol Infect Dis. 1988 Aug;7(4):505–510. doi: 10.1007/BF01962601. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos Y., Toranzo A. E., Barja J. L., Nieto T. P., Villa T. G. Virulence properties and enterotoxin production of Aeromonas strains isolated from fish. Infect Immun. 1988 Dec;56(12):3285–3293. doi: 10.1128/iai.56.12.3285-3293.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabtai Y., Daya-Mishne N. Production, purification, and properties of a lipase from a bacterium (Pseudomonas aeruginosa YS-7) capable of growing in water-restricted environments. Appl Environ Microbiol. 1992 Jan;58(1):174–180. doi: 10.1128/aem.58.1.174-180.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y., Miller K. J. Cloning, expression, and nucleotide sequence of a lipase gene from Pseudomonas fluorescens B52. Appl Environ Microbiol. 1992 Apr;58(4):1402–1407. doi: 10.1128/aem.58.4.1402-1407.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton J., Howard S. P., Buckley J. T. Molecular cloning of a phospholipid-cholesterol acyltransferase from Aeromonas hydrophila. Sequence homologies with lecithin-cholesterol acyltransferase and other lipases. Biochim Biophys Acta. 1988 Mar 25;959(2):153–159. doi: 10.1016/0005-2760(88)90026-4. [DOI] [PubMed] [Google Scholar]
- Wong K. R., Green M. J., Buckley J. T. Extracellular secretion of cloned aerolysin and phospholipase by Aeromonas salmonicida. J Bacteriol. 1989 May;171(5):2523–2527. doi: 10.1128/jb.171.5.2523-2527.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oort M. G., Deveer A. M., Dijkman R., Tjeenk M. L., Verheij H. M., de Haas G. H., Wenzig E., Götz F. Purification and substrate specificity of Staphylococcus hyicus lipase. Biochemistry. 1989 Nov 28;28(24):9278–9285. doi: 10.1021/bi00450a007. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]