Abstract
Whereas the standard immunosuppressive agents foster development of posttransplant lymphoproliferative disorders (PTLDs), the impact of RAD, a macrolide with potent immunosuppressive properties, and other immunosuppressive macrolides on these disorders remains undetermined. We found that RAD had a profound inhibitory effect on in vitro growth of six different PTLD-like Epstein–Barr virus+ lymphoblastoid B cell lines. Similar to normal T cells, RAD blocked cell-cycle progression in PTLD-like B cells in the early (G0/G1) phase. Furthermore, RAD increased the apoptotic rate in such cells. The drug also had a profound inhibitory effect on the growth of PTLD-like Epstein–Barr virus+ B cells xenotransplanted s.c. into SCID mice. The degree of the RAD effect varied among the three B cell lines tested and was proportional to its effects on the cell lines in vitro. In this in vivo xenotransplant model, RAD markedly delayed growth or induced regression of the established tumors. In one line, it was able to eradicate the tumor in four of eight mice. When RAD treatment was initiated before tumor cell injection, a marked inhibition of tumor growth was seen in all three lines. In two of them, the drug prevented tumor establishment in approximately 50% of mice (5/11 and 5/8). In summary, RAD is a potent inhibitor of PTLD-like cells in vitro and in vivo. These findings indicate that, in contrast to the standard immunosuppressive agents, macrolides such as RAD may be effective in prevention and treatment of PTLDs.
Posttransplant lymphoproliferative disorders (PTLDs), which usually represent expansion of B lymphocytes infected with Epstein–Barr virus (EBV), are a life-threatening complication of the immunosuppressive therapy necessary to prevent graft rejection (1, 2). PTLDs comprise a whole spectrum of lymphoproliferative disorders ranging from a polyclonal atypical lymphoid hyperplasia to a monoclonal, overtly malignant B cell lymphoma (1–4). Less advanced forms of PTLDs respond to a decrease in the dose of the immunosuppressive agents (1, 5). However, lowering the drug dose jeopardizes survival of the graft. Furthermore, it is not effective in the more malignant, lymphoma-type cases of PTLDs, which are usually fatal for the graft recipient. Clearly, more effective therapeutic and preventive measures are required to limit the severity and frequency of PTLDs.
RAD (SDZ RAD) is a macrocyclic lactone with potent immunosuppressive and antiproliferative properties (6–10). Like rapamycin, a compound from which RAD was derived by chemical derivation, RAD inhibits growth factor-induced proliferation of hematopoietic as well as nonhematopoietic cells (7). It has been shown earlier for rapamycin that this compound inhibits intracellular signaling events downstream of the receptor for IL-2 and also other cytokine receptors (11, 12) and arrests the cell-cycle progression at the early G1 phase (13, 14).
Herein, we report that RAD has potent inhibitory activity also on PTLD-like, human EBV+ lymphoblastoid B cell lines both in vitro and in vivo. RAD profoundly inhibited in vitro proliferation of such cells and arrested their cell-cycle progression at the early G0/G1 stage. In addition, the compound increased apoptotic rate of the EBV+ B cells. In vivo, it markedly delayed or completely inhibited growth of the EBV+ B cells xenotransplanted into SCID mice, particularly when administered before the cell implantation. RAD was able to eradicate the established tumor in some instances; this effect seemed to be cell line specific and proportional to the drug effect seen in vitro. Clinical implications of these findings in regard to prevention and treatment of PTLDs are discussed.
Materials and Methods
Cell Lines.
All B cell lines used in this study, with the exception of the BC-1 cell line, were lymphoblastoid B cell lines obtained by in vitro infection with EBV of peripheral blood mononuclear cells. Cell lines A1 and A2D6 were obtained from normal, healthy individuals. Cell lines 15A and 20A were obtained from two different patients with low-grade B cell lymphomas with monoclonal cold agglutinins (15). Both lines secreted cold agglutinins with the same specificity as the cold agglutinins found in the patients' serum (15). Furthermore, the 20A cell line showed cytogenetic abnormalities seen in low-grade lymphomas: trisomy 3 and 12 (48, XX, +3, +12; ref. 15). The LCL EBV+ B cell line was obtained from peripheral blood mononuclear cells of a patient with a progressive cutaneous T cell lymphoproliferative disorder (16). BC-1 was derived from a primary effusion B cell lymphoma and, in addition to EBV, harbors HHSV8 virus (17). The other three cell lines, used as controls in the in vitro growth inhibition assay, were HTLV-I (+) T cell lines ATL-2 and C10MJ2 as well as HUT102B derived from patients with adult T cell leukemia/lymphoma; these cell lines had been determined previously by us to be nonresponsive to RAD and rapamycin (18). All cell lines were maintained in humidified incubators at 37°C with 5% CO2 in standard medium: RPMI medium 1640 (GIBCO/BRL) supplemented with 10% (vol/vol) heat-inactivated FBS (BioWhittaker), 1% penicillin/streptomycin/fungizone mixture (GIBCO/BRL), and 2 mmol/liter l-glutamine (GIBCO/BRL).
Inhibition of in Vitro Cell Growth by RAD.
The assay was performed as described (16, 18). Briefly, cell lines were cultured for 32 h in triplicate at 2 × 104 cells per well in the presence of various concentrations of RAD (Novartis Pharma). After pulse with 0.5 μCi [3H]thymidine (New England Nuclear) and culture for the next 18 h, isotope incorporation to the cells was measured. The results of proliferation assays were expressed as the mean radioactivity of triplicate cultures. Standard deviation within the triplicates was <15%.
Detection of Cell-Cycle Inhibition and Apoptosis.
The cell lines to be examined were cultured with several concentrations of RAD (0 to 10 nM) for 24–48 h. The cells were washed with Dulbecco's PBS and stain solution (pH 7.2) containing 3% (wt/vol) polyethylene glycol (molecular weight = 6,000), 50 μg/ml DNA fluorochrome propidium iodide (Calbiochem), annexin V-FITC as described (20), 0.1% Triton X-100 (Sigma), 4 mM citrate buffer (pH 7.8), and 360 units/ml RNase A (Worthington) for 30 min at 37°C. Next, salt solution [pH 7.2; 3% (wt/vol) polyethylene glycol (molecular weight = 6,000)/50 μg/ml propidium iodide/0.1% Triton X-100/0.4 M NaCl] was added, and the cells were incubated at 4°C in the dark for 1 h before flow cytometry analysis (19–21).
Mice.
Immunodeficient 5- to 7-week-old SCID mice (C.B-17 and ICR) were purchased from Taconic Farms, housed at the University of Pennsylvania Animal Facility under pathogen-free conditions in a laminar air flow unit, and supplied with sterile food and water. In the drug tolerability studies, 5- to 7-week-old inbred BALB/c mice (Taconic Farms) were used in addition to the SCID mice.
Establishment of the PTLD-Like Tumors in SCID Mice.
Establishment and passaging of the xenotransplanted lymphoma tumors was performed as described (22–24). To deplete macrophages and natural killer cells and to enhance tumor engraftment, SCID mice were injected i.p. with 30–45 mg/kg of etoposide (Bedford Laboratories, Bedford, OH) 4 days before implantation of the human EBV+ B cell lines (24). Cells (n = 10 million) of each line (see Results) were inoculated into mice either i.p. or s.c. in 200 μl of Dulbecco's PBS (BioWhittaker). Ascites or palpable s.c. tumors developed 3–5 weeks after cell injection. The tumor treatment and growth prevention experiments were performed by using fragments of the established s.c. tumors (22–24). For this purpose, mice were anesthetized with Ketalar (ketamine, Parke-Davis) by i.p. injection of 100 mg/kg. Next, the primary tumor was aseptically removed and freed from necrotic, fatty, and connective tissue and divided into small pieces of roughly equal size, and three or four pieces per mouse were injected s.c. Treatment with RAD of established tumors was started when the tumors reached 5 mm in diameter. In growth prevention experiments, the treatment was initiated 3 days before tumor implantation. Tumor volume in all experiments was determined from the equation, volume = 0.4ab2, where a and b designate, respectively, long and short diameters of the tumor. The transplanted mice were monitored for tumor growth for a period of up to 2 months, and 5 mg/kg of RAD was given once a day by gavage as described (6, 7).
Macroscopic and Microscopic Evaluation of Organs and Xenotransplanted Tumors.
Mice were killed by exposure to forane (isoflurane, Ohmeda, Liberty Place, NJ) on day 29 in the drug toxicity study, on day 40–55 in the tumor growth inhibition study, or when tumors achieved approximately 2 cm in diameter or when ulceration of the skin, signs of severe respiratory distress, weakness, or lethargy appeared. A complete autopsy was performed on all mice at the end of the study regardless of their appearance. Tumor and internal organs (spleen, liver, lung, heart, kidney, small and large intestines, and femoral bone for bone marrow) were fixed in 10% (vol/vol) formalin, paraffin embedded, cut into 0.4-μm sections, transferred to glass slides, and stained with hematoxylin and eosin. Representative tumor fragments were stained immunohistochemically by the standard streptavidin-biotin complex technique with commercially available reagents (Research Genetics, Huntsville, AL) with the following antibodies: anti-CD20 (L-26) and LMP-1 (both from Dako) and Ki-67 (mib1; Immunotech, Westbrook, ME). EBV-encoded RNA was detected with commercially available reagents (Dako).
Results
RAD Inhibits Growth of EBV+ B Cells in Vitro.
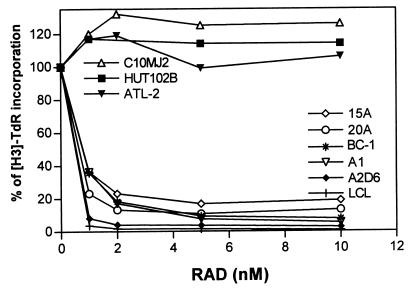
To determine whether RAD can inhibit proliferation of cells mimicking PTLDs, we cultured six different EBV+ B cell lines in the presence of the drug at various concentrations. Three HTLV-I+ T cell lines resistant to RAD (18) were used as controls. As shown in Fig. 1, all PTLD-like EBV+ B cell lines were very sensitive to RAD. A dose of RAD as small as 1 nM produced 60–95% inhibition of growth in all cell lines. This result was comparable to the inhibition of stimulated normal T lymphocytes (7, 25). Some subtle differences in the degree of response were noted among the EBV+ B cell lines. The lines derived from patients with B cell lymphomas (15A, 20A, and BC-1; see Materials and Methods), and thus resembling the more advanced forms of PTLD, tended to show a lower degree of inhibition (80–90%), whereas the lines obtained from normal B cells, and thus mimicking the less advanced types of PTLD, were inhibited more profoundly (90–100%).
Figure 1.
RAD-mediated inhibition of in vitro proliferation of PTLD-like EBV+ B cells. BC-1 is an EBV+/HSV8+ B cell line derived from primary effusion lymphoma. The other cell lines are in vitro EBV-transformed B cell lines derived from patients with low-grade B cell lymphoma (15A and 20A), a patient with T cell lymphoma (LCL), or healthy individuals (A1 and A2D6). HTLV-I+ malignant T cell lines HUT-102, C10MJ, and ATL-2 served as controls. The cell lines were pulsed for 18 h with tritiated thymidine (TdR) after 32 h culture with 0–10 nM of RAD.
RAD Blocks Cell-Cycle Progression in EBV+ B Cells.
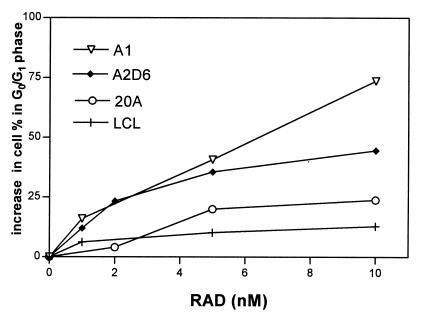
To elucidate at which stage of the cell cycle RAD inhibits the proliferation of PTLD-like B cells, we analyzed the DNA content of the cultured cells by means of flow cytometry. As shown in Fig. 2, treatment with RAD led to an increase in the number of cells in the G0/G1 phase of the cell cycle, indicating an arrest of cell-cycle progression at the early G0/G1 phase. This arrest was observed in all four PTLD-like B cell lines investigated: 20A, A1, A2D6, and LCL. The effect was drug-dose and cell-line-type dependent. Whereas the low RAD doses (1–2 nM) increased the percentage of cells in G0/G1 by 5–25%, the highest dose tested (10 nM) resulted in a 10–70% increase. Percentages of cells in the later phases of the cell cycle (G2/M and S) were diminished proportionally (data not shown). A1 and, to a lesser degree, the A2D6 cell line were particularly sensitive to RAD.
Figure 2.
RAD-mediated inhibition of cell-cycle progression in PTLD-like B cells. Four EBV+ B cell lines were cultured for 48 h with 0–10 nM of RAD, labeled with propidium iodine, and analyzed by flow cytometry.
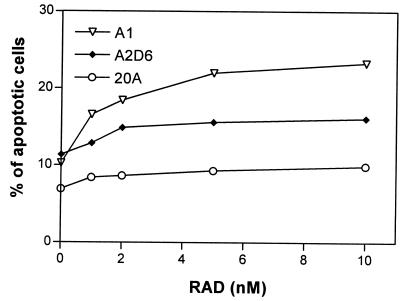
RAD Increases Apoptosis in EBV+ B Cells.
Previous studies have shown that immunosuppressive macrolides as represented by rapamycin can induce or enhance apoptosis stimulated by other agents (26–29). To determine whether RAD affects the apoptotic rate of PTLD-like cells, we tested by flow cytometry the apoptosis-induced cell membrane binding of annexin V in the 20A, A1, and A2D6 cell lines (Fig. 3). Even the lowest RAD dose (1 nM) increased the percentage of apoptotic cells in all cell lines, with some differences resembling the cell growth and cell-cycle inhibition data. In the most sensitive line, A1, RAD resulted in an increase in the number of apoptotic cells from 10 to 16%. RAD led to an a rather moderate increase from 11 to 13% in the A2D6 line and from to 6 to 8% in the 20A line. At the highest dose of RAD (10 nM), 24% of A1 cells, 16% of A2D6 cells, and only 9% of 20A cells were apoptotic.
Figure 3.
RAD-mediated increase in apoptotic rate of PTLD-like B cells. Three EBV+ B cell lines were cultured for 24 h with 0–10 nM of RAD, labeled with propidium iodine and anti-annexin V antibody, and analyzed by flow cytometry.
RAD Tolerability Study.
Previous studies have shown that a minimal effective immunosuppressive dose of RAD was 5 mg/kg/day and >5 mg/kg/day in the rat kidney and heart allotransplant models, respectively, when RAD was used as a single immunosuppressive agent (6, 7). Because there was only very limited experience with RAD in mice, we tested how prolonged exposure to the compound was tolerated by immunocompetent and immunodeficient mice. To this end, we treated a cohort of normal BALB/c mice with a dose of 5 mg/kg/day RAD for 28 days; 10 treated and 5 control untreated mice were used in this study. Subsequently, we also analyzed SCID mice that had been transplanted with human PTLD-like lymphomas and treated with the same dose of RAD for up to 55 days (see below). Seven treated and five control untreated SCID mice were evaluated in this experiment. After the last dose of the drug, all mice were killed, and the following organs were harvested for histopathologic evaluation of possible toxic effects: liver, spleen, kidney, small intestine, large intestine, heart, lung, and femoral bone for bone marrow. None of the treated mice showed any visible signs of drug toxicity. Growth and weight gain were the same for the RAD-treated and the control group. Microscopic evaluation of the organs of all treated and untreated mice revealed no pathologic changes that could be attributed to the drug. We conclude, therefore, that a prolonged exposure to RAD at the dose of 5 mg/kg/day has no adverse effect in the treated mice regardless of their immune status.
Establishment of the Xenotransplant Model of Human PTLD-Like Lymphoma in SCID Mice.
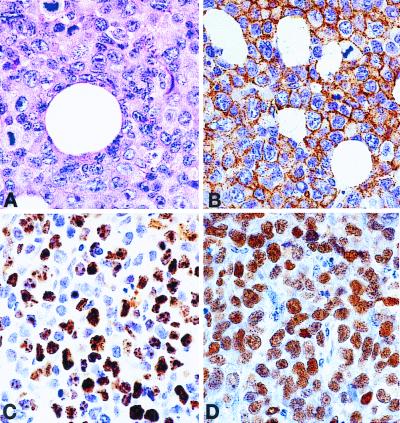
In these experiments, we injected mice with EBV+ B cell lines via two different routes: i.p. and s.c. In the i.p. tumor model, SCID mice (five per group) were inoculated i.p. with 107 cells per line from four cell lines: 15A, 20A, A2D6, and BC-1. After 21–35 days, all mice developed fatal disease with ascites and symptoms of weakness or lethargy. Autopsy revealed extensive tumor infiltrates involving the peritoneal wall, liver, spleen, and kidneys. Microscopic examination of the tumors showed a large cell lymphoma with an infiltrative growth pattern, high mitotic rate, focal and single-cell necrosis, and some degree of plasmacytoid differentiation. Foci of the lymphoma were also present in distant organs such as lungs and bone marrow, indicating hematogenous spread. In the s.c. model, mice (also five per group) were injected with 107 cells from the 15A, 20A, and A1 lines. After 21–32 days, all mice developed tumors at the site of implantation. Autopsy revealed s.c. tumors invading adjacent skeletal muscle and skin. There was no gross evidence of distant spread of the tumor. Microscopic examination revealed the same type of high-grade lymphoma as in the i.p. model. Distant internal organs, mainly liver and lungs, occasionally had small tumor foci. These foci occurred only when the s.c. lymphoma reached a size of at least 1.5 cm in diameter. Immunohistochemical staining confirmed that the tumors were derived from the implanted human EBV+ B lymphocytes. The images of the 20A line shown in Fig. 4 A–D are representative for all three cell lines. Virtually all lymphoma cells were positive for the human B cell marker CD20. Most (50–80%) were positive for cell-cycle related Ki-67 antigen consistent with their high proliferative rate. Staining for EBV-related antigen EBV-encoded RNA1 (EBER1) was universally positive (100% of cells); 20–50% of cells expressed EBV-associated latent membrane protein 1 (LMP-1; not shown). These results confirm that the tumors represent human EBV+ B cell lymphoma corresponding to the monomorphic type of PTLD.
Figure 4.
Morphology and phenotype of the 20A cell line, xenotransplanted into a SCID mouse. (A) Hematoxylin-eosin stain showing large cell lymphoma with high mitotic rate. (B) Immunoperoxidase stain with antibody against human CD20 (B cell antigen) showing cell-membrane staining in all lymphoma cells. (C) Immunoperoxidase stain with an antibody against Ki-67 (cell proliferation-related antigen) showing nuclear staining in 50–80%. (D) In situ hybridization for EBV-encoded RNA1 (EBER1) showing nuclear positivity in all lymphoma cells.
The s.c. lymphoma model had some advantages over the peritoneal model. First, even small lymphomas could be identified easily. Second, the lymphomas of up to 1.5 cm in diameter remained localized, which permitted us to determine the total tumor volume with great accuracy (22). Finally, the s.c. tumor could be transferred simultaneously into several mice by implanting tumor tissue fragments rather than single-cell suspensions (22–24). Such implantation resulted in a fast establishment of tumors with very similar growth characteristics in virtually all recipient mice (22–24). For these reasons, we selected the s.c. model for further studies.
Treatment of Established EBV+ B Cell Tumors.
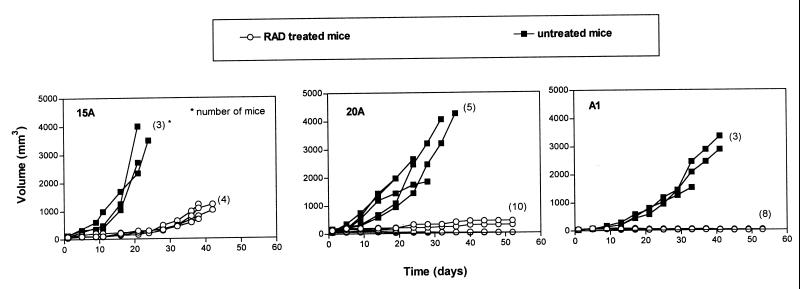
To determine the in vivo effect of RAD on the PTLD-like cells, 15A, 20A, and A1 cell line tumors were implanted into 7–16 SCID mice per cell line. Treatment was initiated once the tumors reached 5 mm in diameter, which corresponds to a volume of 50 mm3. RAD was administered by daily gavage at 5 mg/kg, which was a well tolerated (see above) and effective immunosuppressive (6, 7) dose. As shown in Fig. 5, RAD had a profound inhibitory effect on growth of the xenotransplanted PTLD-like tumors, with visible differences in the degree of response among the three tumors. In mice implanted with 15A tumors, there was a marked drug-induced delay of the tumor growth but no absolute tumor growth inhibition or regression. On day 21, the median tumor volume was approximately 240 mm3 in the treated mice compared with 2,720 mm3 in the control untreated mice. As late as day 38, 15A tumors in the treated mice reached only around 1,000 mm3.
Figure 5.
RAD-mediated inhibition of in vivo growth of PTLD-like B cells; treatment of established tumors. Fragments of tumors derived from EBV+ B cell lines 15A, 20A, and A2D6 were implanted into recipient SCID mice. Treatment with 5 mg/kg/day of the drug was started when the tumors reached 5 mm in diameter (50 mm3 in volume). The numbers of mice per group are shown in parentheses; lines depicting results from some of the mice are superimposed, particularly in the RAD-treated mice.
The effect of RAD on the 20A tumors was even more striking. Whereas on day 19, the control 20A tumors had a median volume of 1,440 mm3, the average treated tumor measured only 50 mm3, which was equal to the initial tumor size. Furthermore, a significant regression in the tumor volume was seen in 6 of 10 treated mice. Accordingly, the median tumor volume on day 53 was only <5 mm3 for this subset.
RAD proved to be the most effective against the A1 cell line. On day 21, the median volume of the treated tumors was 50 mm3, with none of the eight tumors showing any evidence of growth. Mean volume of the untreated tumors on that day was approximately 1,800 mm3. Further treatment resulted in a steady regression in all eight mice. On day 53, the mean tumor volume decreased to <5 mm3, and no lymphoma could be detected in four mice, indicating total tumor eradication. The other four mice had microscopically confirmed residual lymphoma. It is noteworthy that the differences in the in vivo effectiveness of RAD against the 15A, 20A, and A1 tumors paralleled the differences seen in the proliferation (Fig. 1) and the other in vitro assays (Figs. 2 and 3). This observation suggests that cell analysis in vitro may be predictive of the response to RAD in vivo.
Prevention of Tumor Establishment.
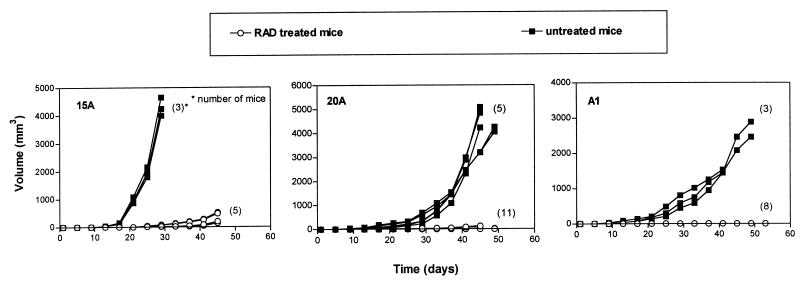
Because RAD as an immunosuppressive agent is administered chronically to transplant patients, its main therapeutic impact on PTLDs may be to inhibit their development rather than to treat clinically symptomatic cases. To test whether RAD can prevent establishment of the PTLD-like lymphomas, daily treatment with 5 mg/kg of the drug was initiated 3 days before tumor implantation. As shown in Fig. 6, RAD proved to be extremely effective in this model. For 15A tumors, which are the least sensitive to RAD (Fig. 5), treatment profoundly delayed tumor growth but was unable to prevent tumor establishment. On day 25, the treated tumors measured on average 38 mm3, and the untreated tumors measured approximately 1,940 mm3. On day 45, the median volume of the treated tumors was 480 mm3.
Figure 6.
RAD-mediated inhibition of in vivo growth of a malignant PTLD-like B cells; prevention of tumor growth. Fragments of tumors derived from EBV+ B cell lines 15A, 20A, and A2D6 were implanted into recipient SCID mice. The treatment with 5 mg/kg/day of the drug was started 3 days before the tumor implantation. The numbers of mice per group are shown in parentheses; lines depicting results from some of the mice are superimposed.
Of 11 20A tumors, 6 were nondetectable on day 25; the median volume of the remaining 5 was 20 mm3. The average untreated tumor measured 210 mm3 on that day. On day 45, none of the treated tumors exceeded 150 mm3, and the average untreated tumor measured approximately 4,200 mm3. Five treated mice showed no signs of tumor; microscopic evaluation of the implantation site revealed no malignant cells.
Finally, in mice with A1 cells, no tumor could be detected as late as on day 29 in any of the eight treated mice; the average tumor in the untreated mice measured 580 mm3. On day 53, only three mice showed small (<5 mm3), histologically confirmed lymphomas. The remaining five mice showed no evidence of tumor.
Discussion
New treatment modalities are needed to inhibit development and improve cure rate of PTLDs. An ideal anti-PTLD drug would play a double role of preventing graft rejection and, at the same time, inhibiting development and growth of PTLD. Should PTLD develop despite the treatment, an increase, rather than decrease in the drug dose as currently done with the standard immunosuppressive drugs, might be effective. This approach of increasing the drug dose would have an additional advantage of not jeopardizing survival of the graft. Herein, we present in vitro and in vivo data that indicate that RAD, a macrolide immunosuppressant, may play such a double role as an antirejection and anti-PTLD drug. RAD profoundly suppressed in vitro proliferation, arrested cell-cycle progression at the early G0/G1 stage, and increased apoptotic rate in PTLD-like human EBV+ B cells. In SCID mice xenotransplanted with three different PTLD-like B cell tumors, RAD markedly inhibited growth of these tumors, particularly if given before the tumor implantation. With the A1 tumors, total eradication of already established tumors was achieved in four of eight mice. In the other two lines tested (20A and A1), RAD completely prevented establishment of tumors in approximately 50% of mice (5/11 and 5/8, respectively). The anti-EBV+ B cell tumor effect of RAD contrasts with the standard immunosuppressive agents cyclosporin and tacrolimus, which have been suggested recently to enhance outgrowth of EBV+ B cells, not only indirectly by suppressing an immune response against such cells, but also directly by protecting them from the effects of proapoptotic signals (30).
We consider our results particularly encouraging, because the 15A and 20A lines were derived from lymphoma cells (15) and as such seem to correspond to advanced, clinically aggressive PTLD. It is plausible, therefore, that other, less malignant forms of PTLD, which comprise the majority of clinical cases, should be even more sensitive to RAD. The fact that the A1 cell line obtained from a normal individual was more sensitive than 15A and 20A to RAD supports this assumption. Our data also suggest that monotherapy with RAD may not be sufficient to eradicate some established, overtly malignant PTLD tumors, and combination therapy with conventional chemotherapeutic drugs might need to be considered in such clinical settings (28, 31). Alternatively, adoptive transfer of in vitro generated EBV-specific cytotoxic T cells, recently shown to be effective in patients at risk or with an overt PTLD (32, 33), may be used together with RAD.
Although inhibition of cell growth as measured by thymidine incorporation (Fig. 1) and cell-cycle progression (Fig. 2) seems to be the main mode of RAD action on the EBV+ B cells, our finding that RAD also increases apoptotic rate in such cells (Fig. 3) suggests that programmed cell death might also play a role in the antitumor activity of the drug. This proapoptotic effect may be particularly important in treatment of already established PTLD tumors where inhibition of tumor growth alone may not be sufficient to achieve complete tumor regression. Also, our finding that only a prolonged exposure to RAD led to marked regression or elimination of many tumors (Fig. 5) suggests that a similar extended treatment may be required to eradicate established PTLDs.
Because the PTLD-like B cell lines sensitive to RAD were all EBV+, the potential role, if any, of the virus in mediating this sensitivity needs to be explored. EBV encodes or induces in the target cells several proteins capable of activating cytokine signaling pathways (34). The membrane-anchored viral LMP-1, which is the best characterized, uses the TRAF signaling pathway of the tumor necrosis factor receptor family (35–37). It is interesting in this context, that signaling via CD40, which belongs to the family and shares several features with LMP-1 including signaling via TRAF3 protein (38, 39), has been shown recently to be inhibited by rapamycin (12). However, our immunohistochemical analysis indicates that LMP-1 is expressed only by a subset of EBV+ B cells, which suggests that LMP-1 may not be critical for growth of the PTLD-like cells and their sensitivity to RAD. EBV also encodes two other, related membrane proteins, LMP-2A and LMP-2B, but they also do not seem to be essential for in vivo growth of EBV+ B cells (40). Alternatively, RAD might inhibit signaling mediated by cytokines induced by EBV in the target cells, such as tumor necrosis factor-α and tumor necrosis factor-β (34). However, an autocrine role for these or any other cytokine(s) in the EBV+ B cells remains to be established.
In summary, our data show that RAD has a potent inhibitory effect on EBV+ B lymphocytes in vitro and in vivo. Therefore, it may be effective in treatment and prevention of PTLDs in transplant patients.
Acknowledgments
The study was supported in part by a grant from Novartis Pharma and by National Cancer Institute Grant CA76627.
Abbreviations
- PTLD
posttransplant lymphoproliferative disorder
- EBV
Epstein–Barr virus
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.080068597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.080068597
References
- 1.Morrison V, Dunn D L, Manivel C, Gajl-Peczalska K J, Peterson B A. Am J Med. 1994;97:14–24. doi: 10.1016/0002-9343(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 2.Warnke R A, Weiss L M, Chan J K C, Cleary M L, Dorfman R F. Armed Forces Inst Pathol Fascicle. 1995;14:531–535. [Google Scholar]
- 3.Curtis R E, Travis L B, Rowlings P A, Socié G, Douglas W, Kingma D W, Banks P M, Jaffe E S, Sale G E, Horowitz M M, et al. Blood. 1994;94:2208–2216. [PubMed] [Google Scholar]
- 4.Harris N L, Ferry J A, Swierdlow S H. Semin Diagn Pathol. 1997;14:8–14. [PubMed] [Google Scholar]
- 5.Gummert J F, Ikonen T, Morris R E. J Am Soc Nephrol. 1999;10:1366–1380. doi: 10.1681/ASN.V1061366. [DOI] [PubMed] [Google Scholar]
- 6.Schuurman H J, Cottens S, Fuchs S, Joergensen J, Meerloo T, Sedrani R, Tanner M, Zenke G, Schuler W. Transplantation. 1997;64:32–35. doi: 10.1097/00007890-199707150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Schuler W, Sedrani R, Cottens S, Haberlin B, Schulz M, Schuurman H J, Zenke G, Zerwes H G, Schreier M H. Transplantation. 1997;64:36–42. doi: 10.1097/00007890-199707150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Sedrani R, Cottens S, Kallen J, Schuler W. Transplant Proc. 1998;30:2192–2194. doi: 10.1016/s0041-1345(98)00587-9. [DOI] [PubMed] [Google Scholar]
- 9.Schuurman H J, Schuler W, Ringers J, Jonker M. Transplant Proc. 1998;30:2198–2199. doi: 10.1016/s0041-1345(98)00589-2. [DOI] [PubMed] [Google Scholar]
- 10.Hausen B, Boeke K, Berry G J, Segarra I T, Christians U, Morri R E. J Heart Lung Transplant. 1999;18:150–159. doi: 10.1016/s1053-2498(98)00020-5. [DOI] [PubMed] [Google Scholar]
- 11.Seghal S N. Clin Biochem. 1998;31:335–340. doi: 10.1016/s0009-9120(98)00045-9. [DOI] [PubMed] [Google Scholar]
- 12.Sakata A, Kuwahara K, Ohmura T, Inui S, Sakaguchi N. Immunol Lett. 1999;68:301–319. doi: 10.1016/s0165-2478(99)00053-x. [DOI] [PubMed] [Google Scholar]
- 13.Terada N, Lucas J J, Szepesi A, Franklin R A, Domenico J, Gelfand E W. J Cell Physiol. 1993;154:7–15. doi: 10.1002/jcp.1041540103. [DOI] [PubMed] [Google Scholar]
- 14.Flanagan W M, Crabtree G R. Ann NY Acad Sci. 1993;696:31–37. doi: 10.1111/j.1749-6632.1993.tb17139.x. [DOI] [PubMed] [Google Scholar]
- 15.Silberstein L E, Jefferies L C, Goldman J, Friedman D, Moore J S, Nowell P C, Roelcke D, Pruzanski W, Roudier J, Silverman G J. Blood. 1991;78:2372–2386. [PubMed] [Google Scholar]
- 16.Zhang Q, Nowak I, Vonderheid E C, Rook A H, Kadin M E, Nowell P C, Shaw L M, Wasik M A. Proc Natl Acad Sci USA. 1996;93:9148–9153. doi: 10.1073/pnas.93.17.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cesarman E, Moore P S, Rao P H, Inghirami G, Knowles D M, Chang Y. Blood. 1995;86:2708–2714. [PubMed] [Google Scholar]
- 18.Zhang Q, Lee B, Korecka M, Li G, Weyland C, Eck S, Gessain A, Arima N, Shaw L, Luger S, et al. Leukemia Res. 1999;23:373–384. doi: 10.1016/s0145-2126(98)00173-8. [DOI] [PubMed] [Google Scholar]
- 19.Kawamata S, Sakaida H, Hori T, Maeda M, Uchiyama T. Blood. 1998;91:561–569. [PubMed] [Google Scholar]
- 20.Pepper C, Thomas A, Tucker H, Hoy T, Bentley P. Leuk Res. 1998;22:439–444. doi: 10.1016/s0145-2126(98)00013-7. [DOI] [PubMed] [Google Scholar]
- 21.Douglas R S, Pletcher C H, Jr, Nowell P C, Moore J S. Cytometry. 1998;32:57–65. doi: 10.1002/(sici)1097-0320(19980501)32:1<57::aid-cyto8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 22.Wasik M A, Sioutos N, Tuttle M, Butmarc J R, Kaplan W, Kadin M E. Am J Pathol. 1994;144:1089–1097. [PMC free article] [PubMed] [Google Scholar]
- 23.Pasqualucci L, Wasik M A, Teicher B, Flenghi L, Bolognesi A, Stirpe F, Polito L, Falini B, Kadin M E. Blood. 1995;85:2139–2146. [PubMed] [Google Scholar]
- 24.Visonneau S, Cesano A, Torosian M H, Miller E J, Santoli D. Am J Pathol. 1998;152:1299–1311. [PMC free article] [PubMed] [Google Scholar]
- 25.Bohler T, Waiser J, Budde K, Lichter S, Jauho A, Fritsche L, Korn A, Neumayer H H. Transplant Proc. 1998;30:2195–2197. doi: 10.1016/s0041-1345(98)00588-0. [DOI] [PubMed] [Google Scholar]
- 26.Gottschalk A R, Boise L H, Thompson C B, Quintans J. Proc Natl Acad Sci USA. 1994;91:7350–7354. doi: 10.1073/pnas.91.15.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muthukkumar S, Ramesh T M, Bondada S. Transplantation. 1995;60:264–270. doi: 10.1097/00007890-199508000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y, Frankel A, Radvanyi L G, Penn L Z, Miller R G, Mills G B. Cancer Res. 1995;55:1982–1988. [PubMed] [Google Scholar]
- 29.Hosoi H, Dilling M B, Shikata T, Liu L N, Shu L, Ashmun R A, Germain G S, Abraham R T, Houghton P J. Cancer Res. 1999;59:886–894. [PubMed] [Google Scholar]
- 30.Beatty P R, Krams S M, Esqiuvel C O, Martinez O M. Transplantation. 1998;65:1248–1255. doi: 10.1097/00007890-199805150-00017. [DOI] [PubMed] [Google Scholar]
- 31.Miki T, Goller A, Rao A, Wang X, Yin W Y, Tandin A, Fung J J, Starzl T E, Valdivia L A. Transplant Proc. 1998;30:1091–1092. doi: 10.1016/s0041-1345(98)00166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khanna R, Bell S, Sherritt M, Galbraith A, Burrows S R, Rafter L, Clarke B, Slaughter R, Falk M C, Douglass J, et al. Proc Natl Acad Sci USA. 1999;96:10391–10396. doi: 10.1073/pnas.96.18.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gustafsson A, Levitsky V, Zou J-Z, Frisan T, Dalianis T, Ljungman P, Ringden O, Winiarski J, Ernberg I, Masucci M G. Blood. 2000;95:807–814. [PubMed] [Google Scholar]
- 34.Rochford R, Cannon M J, Sabbe R E, Adusumilli K, Picchio G, Glynn J M, Noonan D J, Mosier D E, Hobbs M V. Viral Immunol. 1997;10:183–195. doi: 10.1089/vim.1997.10.183. [DOI] [PubMed] [Google Scholar]
- 35.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 36.Devergne O, McFarland E C, Mosialos G, Izumi K M, Ware C F, Kieff E. J Virol. 1998;72:7900–7908. doi: 10.1128/jvi.72.10.7900-7908.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liebowitz D. N Eng J Med. 1998;338:1413–1421. doi: 10.1056/NEJM199805143382003. [DOI] [PubMed] [Google Scholar]
- 38.Eliopoulos A G, Dawson C W, Mosialos G, Floettmann J E, Rowe M, Armitage R J, Dawson J, Zapata J M, Kerr D J, Wakelam M J, et al. Oncogene. 1996;13:2243–2254. [PubMed] [Google Scholar]
- 39.Pullen S S, Miller H G, Everdeen D S, Dang T T A, Crute J J, Kehry M R. Biochemistry. 1998;37:11836–11845. doi: 10.1021/bi981067q. [DOI] [PubMed] [Google Scholar]
- 40.Rochford R, Miller C L, Cannon M J, Izumi K M, Kieff E, Longnecker R. Arch Virol. 1997;142:707–720. doi: 10.1007/s007050050113. [DOI] [PubMed] [Google Scholar]