Abstract
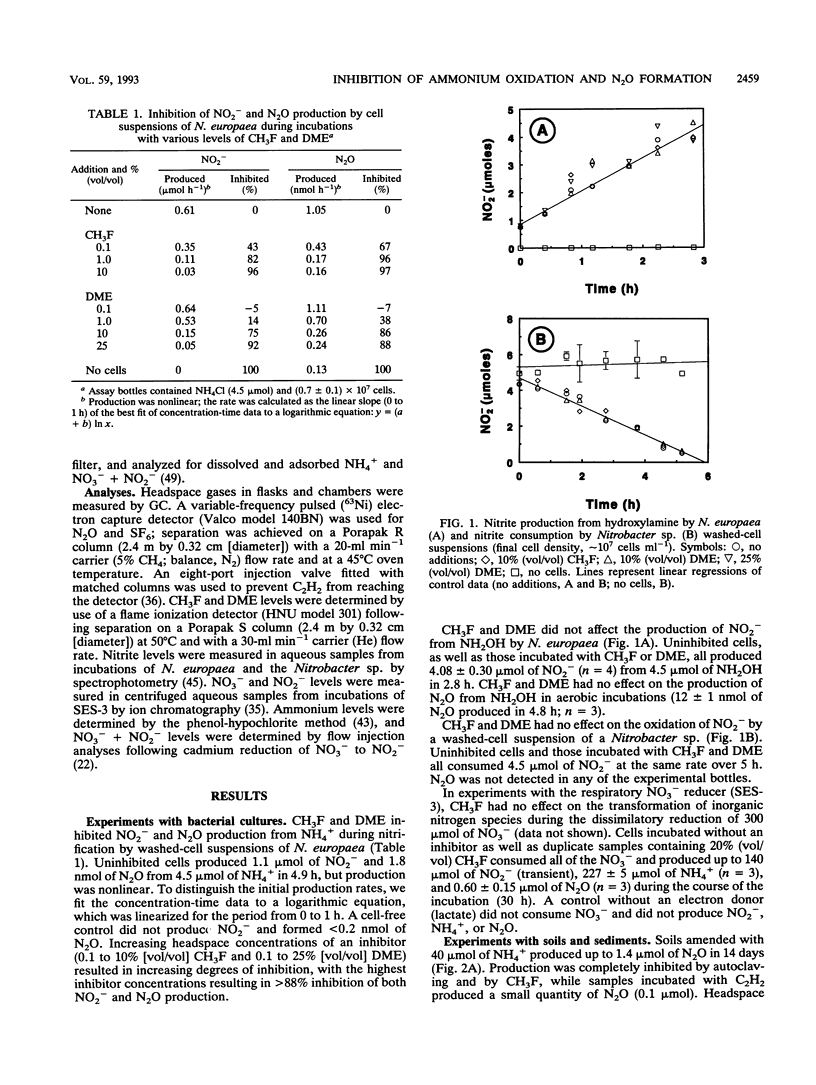
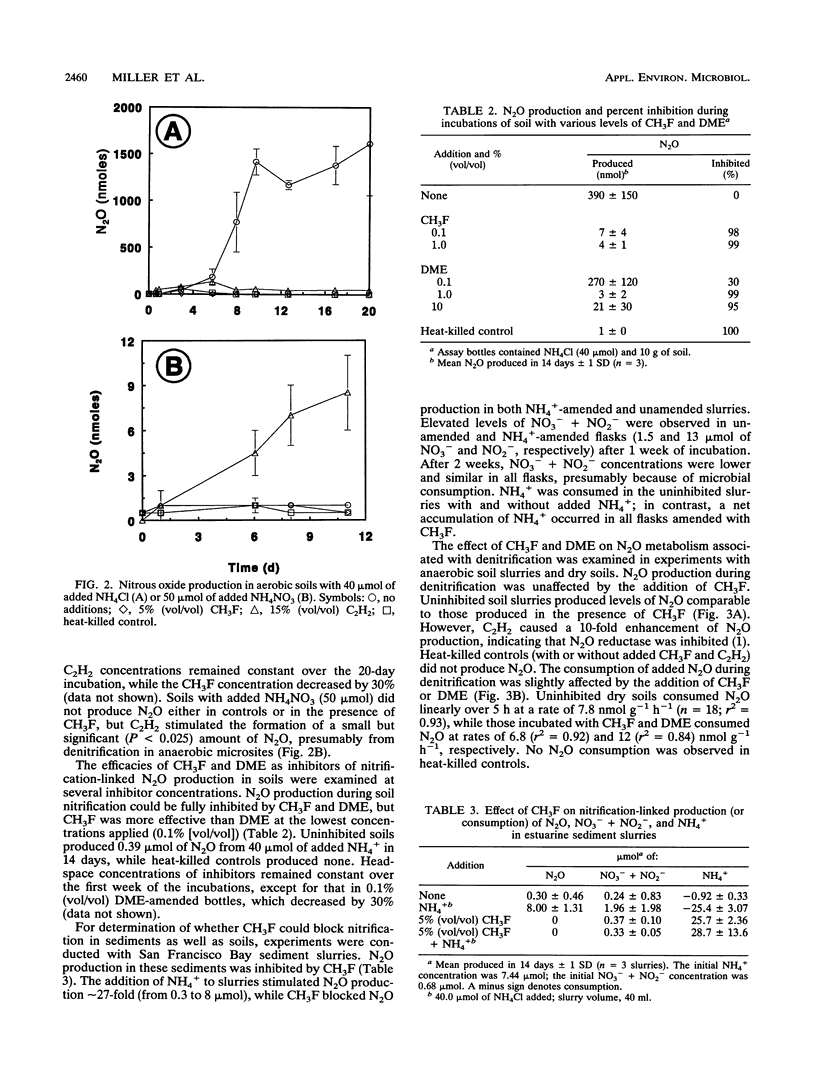
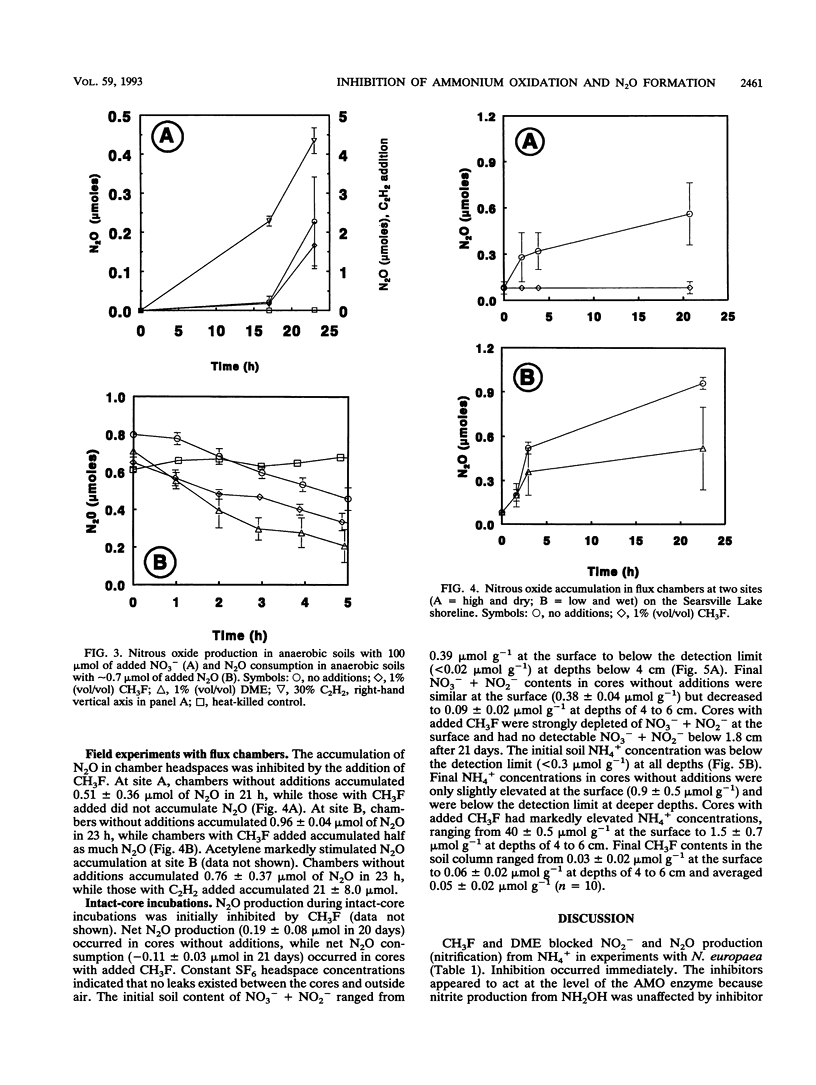
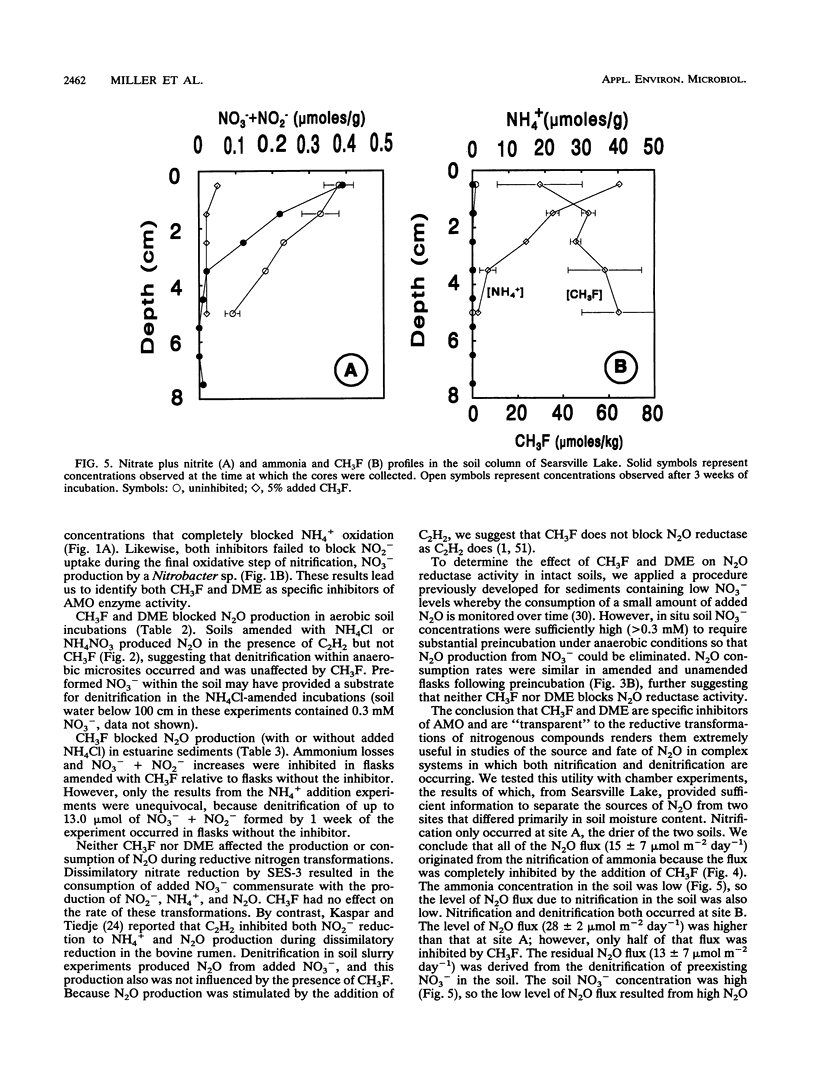
Methyl fluoride (CH3F) and dimethyl ether (DME) inhibited nitrification in washed-cell suspensions of Nitrosomonas europaea and in a variety of oxygenated soils and sediments. Headspace additions of CH3F (10% [vol/vol]) and DME (25% [vol/vol]) fully inhibited NO2- and N2O production from NH4+ in incubations of N. europaea, while lower concentrations of these gases resulted in partial inhibition. Oxidation of hydroxylamine (NH2OH) by N. europaea and oxidation of NO2- by a Nitrobacter sp. were unaffected by CH3F or DME. In nitrifying soils, CH3F and DME inhibited N2O production. In field experiments with surface flux chambers and intact cores, CH3F reduced the release of N2O from soils to the atmosphere by 20- to 30-fold. Inhibition by CH3F also resulted in decreased NO3- + NO2- levels and increased NH4+ levels in soils. CH3F did not affect patterns of dissimilatory nitrate reduction to ammonia in cell suspensions of a nitrate-respiring bacterium, nor did it affect N2O metabolism in denitrifying soils. CH3F and DME will be useful in discriminating N2O production via nitrification and denitrification when both processes occur and in decoupling these processes by blocking NO2- and NO3- production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balderston W. L., Sherr B., Payne W. J. Blockage by acetylene of nitrous oxide reduction in Pseudomonas perfectomarinus. Appl Environ Microbiol. 1976 Apr;31(4):504–508. doi: 10.1128/aem.31.4.504-508.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender M. L., Fanning K. A., Froelich P. N., Heath G. R., Maynard V. Interstitial nitrate profiles and oxidation of sedimentary organic matter in the eastern equatorial atlantic. Science. 1977 Nov 11;198(4317):605–609. doi: 10.1126/science.198.4317.605. [DOI] [PubMed] [Google Scholar]
- Binnerup S. J., Jensen K., Revsbech N. P., Jensen M. H., Sørensen J. Denitrification, dissimilatory reduction of nitrate to ammonium, and nitrification in a bioturbated estuarine sediment as measured with N and microsensor techniques. Appl Environ Microbiol. 1992 Jan;58(1):303–313. doi: 10.1128/aem.58.1.303-313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmer A. M., Bremner J. M., Schmidt E. L. Production of nitrous oxide by ammonia-oxidizing chemoautotrophic microorganisms in soil. Appl Environ Microbiol. 1980 Dec;40(6):1060–1066. doi: 10.1128/aem.40.6.1060-1066.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner J. M., Blackmer A. M. Nitrous oxide: emission from soils during nitrification of fertilizer nitrogen. Science. 1978 Jan 20;199(4326):295–296. doi: 10.1126/science.199.4326.295. [DOI] [PubMed] [Google Scholar]
- Bédard C., Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989 Mar;53(1):68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. A., Swank W. T., Perry T. O. Distinguishing between Nitrification and Denitrification as Sources of Gaseous Nitrogen Production in Soil. Appl Environ Microbiol. 1986 Dec;52(6):1280–1286. doi: 10.1128/aem.52.6.1280-1286.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone M. K., Firestone R. B., Tiedje J. M. Nitrous oxide from soil denitrification: factors controlling its biological production. Science. 1980 May 16;208(4445):749–751. doi: 10.1126/science.208.4445.749. [DOI] [PubMed] [Google Scholar]
- Goreau T. J., Kaplan W. A., Wofsy S. C., McElroy M. B., Valois F. W., Watson S. W. Production of NO(2) and N(2)O by Nitrifying Bacteria at Reduced Concentrations of Oxygen. Appl Environ Microbiol. 1980 Sep;40(3):526–532. doi: 10.1128/aem.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar H. F., Tiedje J. M. Dissimilatory reduction of nitrate and nitrite in the bovine rumen: nitrous oxide production and effect of acetylene. Appl Environ Microbiol. 1981 Mar;41(3):705–709. doi: 10.1128/aem.41.3.705-709.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingensmith K. M., Alexander V. Sediment nitrification, denitrification, and nitrous oxide production in a deep arctic lake. Appl Environ Microbiol. 1983 Nov;46(5):1084–1092. doi: 10.1128/aem.46.5.1084-1092.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike I., Hattori A. Denitrification and ammonia formation in anaerobic coastal sediments. Appl Environ Microbiol. 1978 Feb;35(2):278–282. doi: 10.1128/aem.35.2.278-282.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. G., Oremland R. S., Paulsen S. Measurement of nitrous oxide reductase activity in aquatic sediments. Appl Environ Microbiol. 1986 Jan;51(1):18–24. doi: 10.1128/aem.51.1.18-24.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio T., Koike I., Hattori A. Estimates of denitrification and nitrification in coastal and estuarine sediments. Appl Environ Microbiol. 1983 Feb;45(2):444–450. doi: 10.1128/aem.45.2.444-450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Culbertson C. W. Evaluation of methyl fluoride and dimethyl ether as inhibitors of aerobic methane oxidation. Appl Environ Microbiol. 1992 Sep;58(9):2983–2992. doi: 10.1128/aem.58.9.2983-2992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Umberger C., Culbertson C. W., Smith R. L. Denitrification in san francisco bay intertidal sediments. Appl Environ Microbiol. 1984 May;47(5):1106–1112. doi: 10.1128/aem.47.5.1106-1112.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons P. A. Variations between strains of Drosophila melanogaster and D. simulans in giving offspring in interspecific crosses. Can J Genet Cytol. 1972 Mar;14(1):77–80. doi: 10.1139/g72-010. [DOI] [PubMed] [Google Scholar]
- Payne W. J. Reduction of nitrogenous oxides by microorganisms. Bacteriol Rev. 1973 Dec;37(4):409–452. doi: 10.1128/br.37.4.409-452.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie G. A., Nicholas D. J. Identification of the sources of nitrous oxide produced by oxidative and reductive processes in Nitrosomonas europaea. Biochem J. 1972 Mar;126(5):1181–1191. doi: 10.1042/bj1261181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg N. A., Blum J. S., Hochstein L., Oremland R. S. Nitrate is a preferred electron acceptor for growth of freshwater selenate-respiring bacteria. Appl Environ Microbiol. 1992 Jan;58(1):426–428. doi: 10.1128/aem.58.1.426-428.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


