Abstract
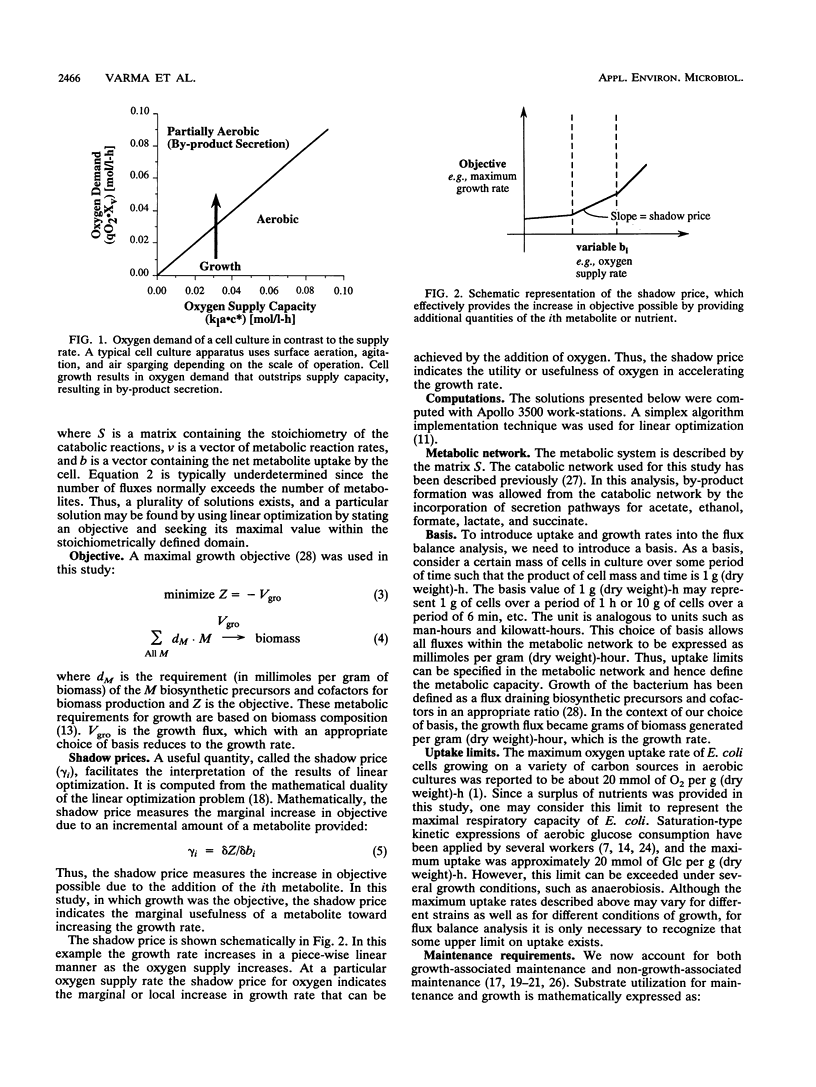
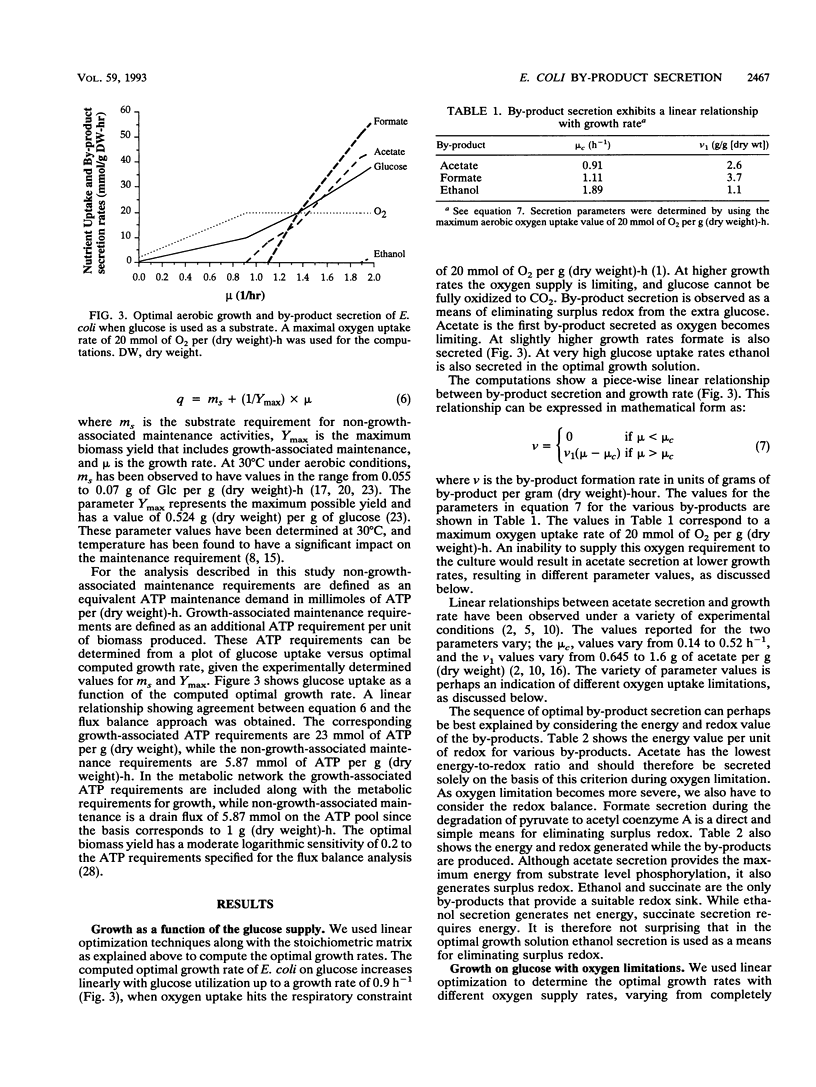
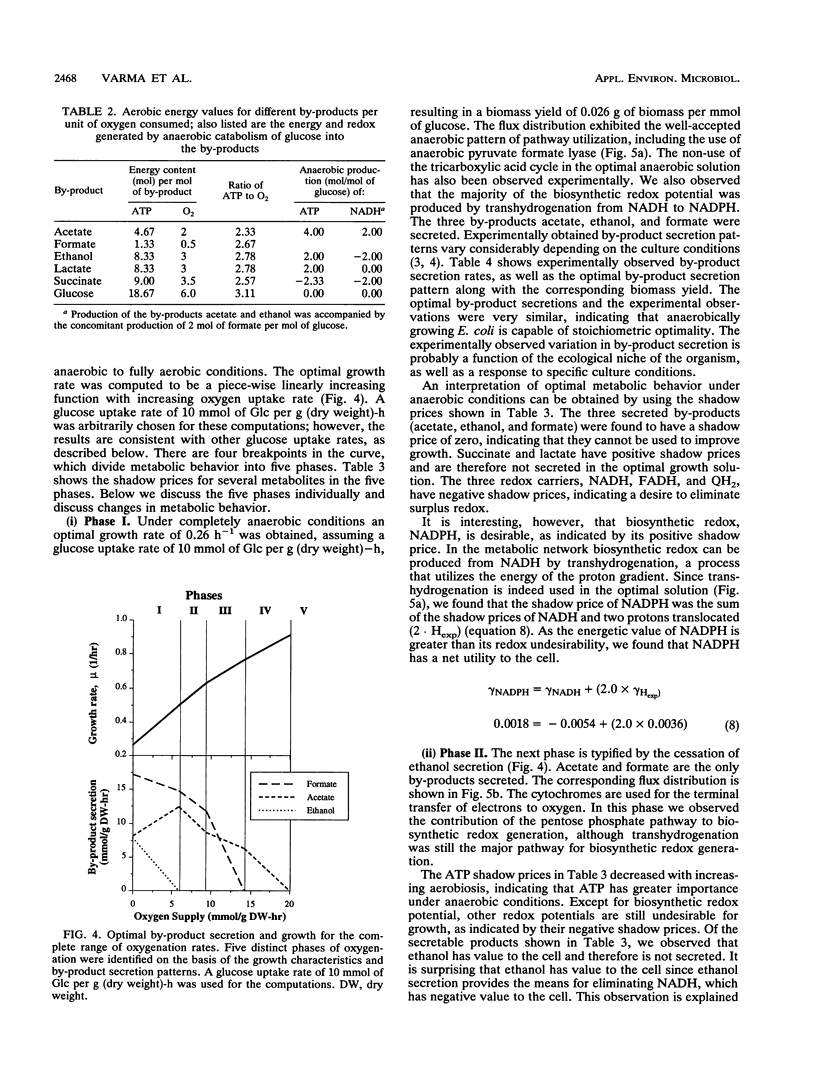
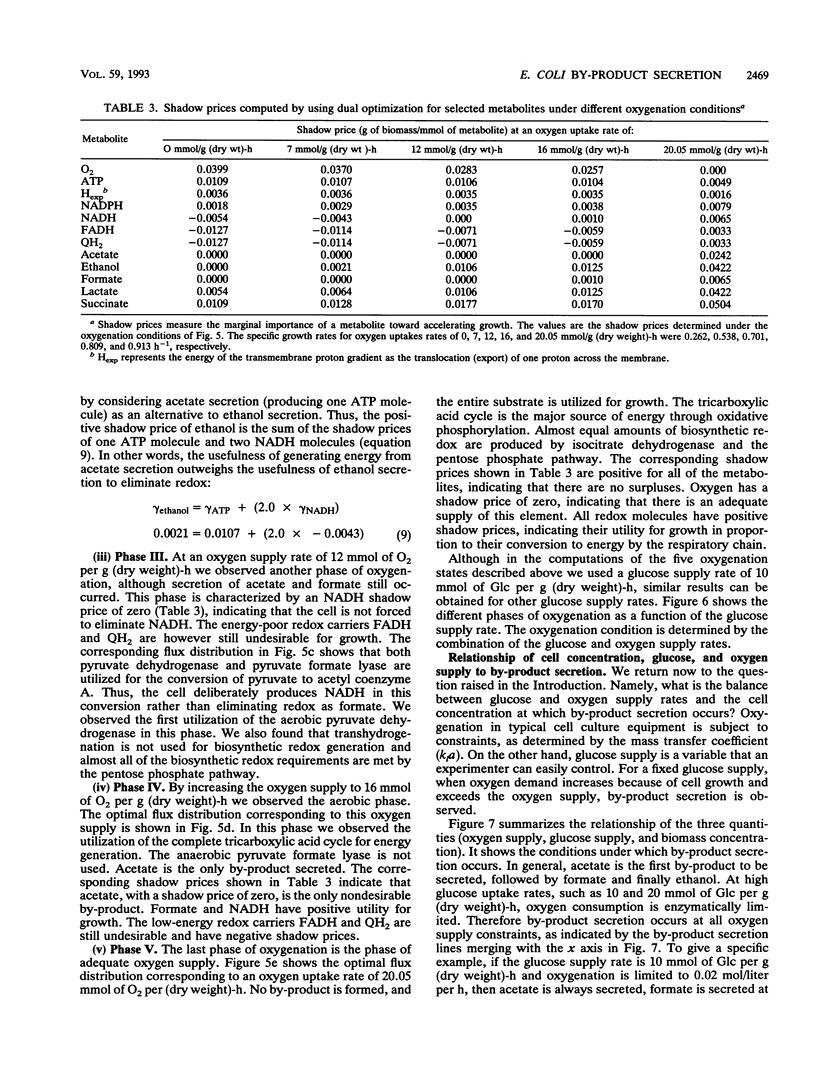
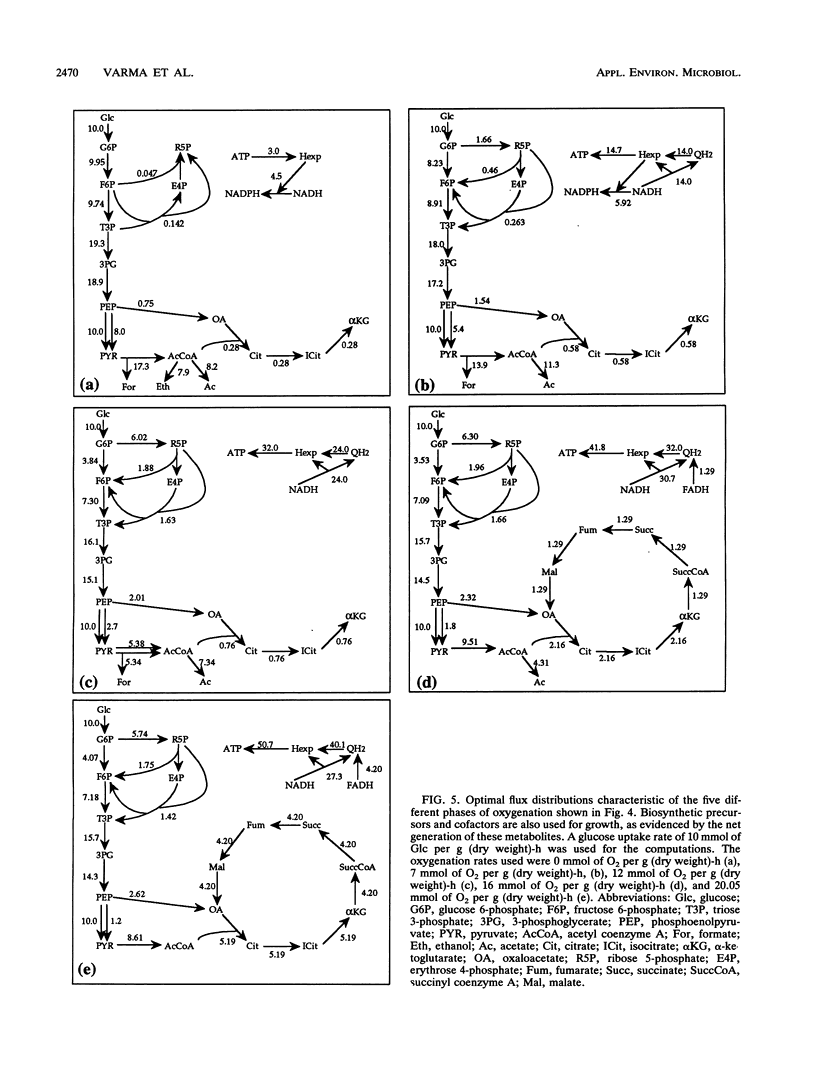
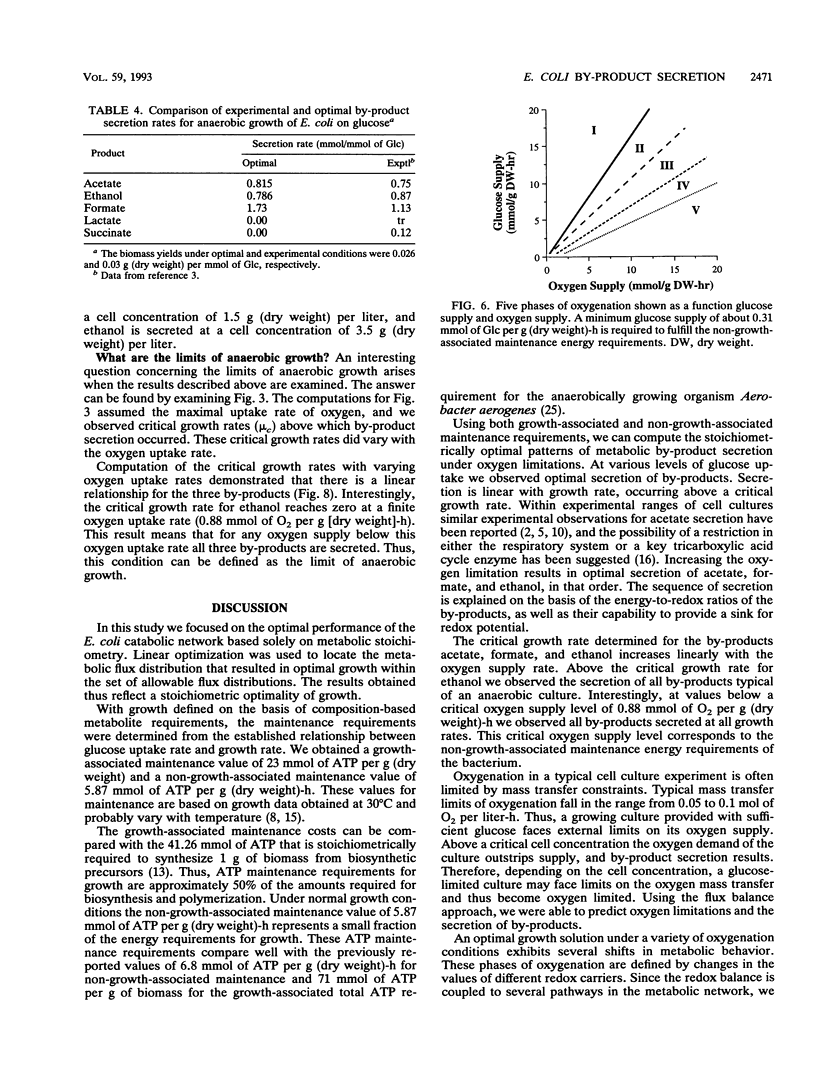
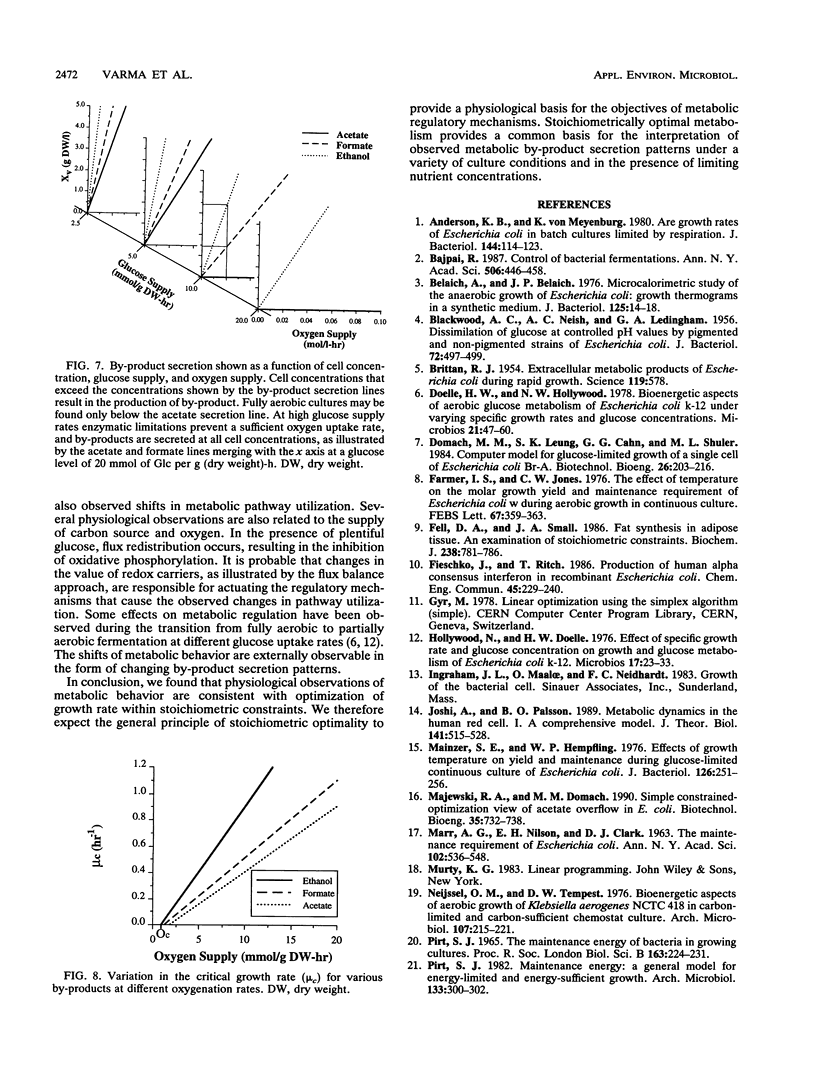
Metabolic by-product secretion is commonly observed in oxygen-limited cultures. Oxygen limitations occur because of limits in the capacity of the respiratory system or because of the oxygenation limits of the cultivation method used. The latter restriction is of considerable practical importance since it results in a critical cell concentration above which oxygenation is insufficient, leading to by-product secretion. In this study we used a flux balance approach to determine optimal metabolic performance of Escherichia coli under variable oxygen limitations. This method uses linear optimization to find optimal metabolic flux patterns with respect to cell growth. Cell growth was defined as precursor requirements on the basis of a composition analysis. A growth-associated maintenance requirement of 23 mmol of ATP per g of biomass and a non-growth-associated maintenance value of 5.87 mmol at ATP per g (dry weight)-h were incorporated on the basis of a comparison with experimental data. From computations of optimal growth increased oxygen limitations were found to result in the secretion of acetate, formate, and ethanol in that order. Consistent with the experimental data in the literature, by-product secretion rates increased linearly with the growth rate. The computed optimal growth under increasing oxygen limitation revealed four critical growth rates at which changes in the by-product secretion pattern were observed. Concomitant with by-product secretion under oxygen limitations were changes in metabolic pathway utilization. The shifts in metabolism were characterized by changes in the metabolic values (computed as shadow prices) of the various redox carriers. The redox potential was thus identified as a likely trigger that leads to metabolic shifts.2+ ă
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen K. B., von Meyenburg K. Are growth rates of Escherichia coli in batch cultures limited by respiration? J Bacteriol. 1980 Oct;144(1):114–123. doi: 10.1128/jb.144.1.114-123.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACKWOOD A. C., LEDINGHAM G. A., NEISH A. C. Dissimilation of glucose at controlled pH values by pigmented and non-pigmented strains of Escherichia coli. J Bacteriol. 1956 Oct;72(4):497–499. doi: 10.1128/jb.72.4.497-499.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajpai R. Control of bacterial fermentations. Ann N Y Acad Sci. 1987;506:446–458. doi: 10.1111/j.1749-6632.1987.tb23840.x. [DOI] [PubMed] [Google Scholar]
- Belaich A., Belaich J. P. Microcalorimetric study of the anaerobic growth of Escherichia coli: growth thermograms in a synthetic medium. J Bacteriol. 1976 Jan;125(1):14–18. doi: 10.1128/jb.125.1.14-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doelle H. W., Hollywood N. W. Bioenergetic aspects of aerobic glucose metabolism of Escherichia coli K-12 under varying specific growth rates and glucose concentrations. Microbios. 1978;21(83):47–60. [PubMed] [Google Scholar]
- Farmer I. S., Jones C. W. The effect of temperature on the molar growth yield and maintenance requirement of Escherichia coli W during aerobic growth in continuous culture. FEBS Lett. 1976 Sep 1;67(3):359–363. doi: 10.1016/0014-5793(76)80564-9. [DOI] [PubMed] [Google Scholar]
- Fell D. A., Small J. R. Fat synthesis in adipose tissue. An examination of stoichiometric constraints. Biochem J. 1986 Sep 15;238(3):781–786. doi: 10.1042/bj2380781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollywood N., Doelle H. W. Effect of specific growth rate and glucose concentration on growth and glucose metabolism of Escherichia coli K-12. Microbios. 1976;17(67):23–33. [PubMed] [Google Scholar]
- Joshi A., Palsson B. O. Metabolic dynamics in the human red cell. Part I--A comprehensive kinetic model. J Theor Biol. 1989 Dec 19;141(4):515–528. doi: 10.1016/s0022-5193(89)80233-4. [DOI] [PubMed] [Google Scholar]
- Mainzer S. E., Hempfling W. P. Effects of growth temperature on yield and maintenance during glucose-limited continuous culture of Escherichia coli. J Bacteriol. 1976 Apr;126(1):251–256. doi: 10.1128/jb.126.1.251-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijssel O. M., Tempest D. W. Bioenergetic aspects of aerobic growth of Klebsiella aerogenes NCTC 418 in carbon-limited and carbon-sufficient chemostat culture. Arch Microbiol. 1976 Mar 19;107(2):215–221. doi: 10.1007/BF00446843. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. Maintenance energy: a general model for energy-limited and energy-sufficient growth. Arch Microbiol. 1982 Dec 3;133(4):300–302. doi: 10.1007/BF00521294. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- SCHULZE K. L., LIPE R. S. RELATIONSHIP BETWEEN SUBSTRATE CONCENTRATION, GROWTH RATE, AND RESPIRATION RATE OF ESCHERICHIA COLI IN CONTINUOUS CULTURE. Arch Mikrobiol. 1964 Apr 2;48:1–20. doi: 10.1007/BF00406595. [DOI] [PubMed] [Google Scholar]
- Savinell J. M., Palsson B. O. Network analysis of intermediary metabolism using linear optimization. I. Development of mathematical formalism. J Theor Biol. 1992 Feb 21;154(4):421–454. doi: 10.1016/s0022-5193(05)80161-4. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. W. Determination of the efficiency of oxidative phosphorylation in continuous cultures of Aerobacter aerogenes. Arch Microbiol. 1975 Mar 10;102(3):187–192. doi: 10.1007/BF00428367. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. W. Energetic aspects of anaerobic growth of Aerobacter aerogenes in complex medium. Arch Microbiol. 1976 Dec 1;111(1-2):21–23. doi: 10.1007/BF00446545. [DOI] [PubMed] [Google Scholar]
- Watson M. R. A discrete model of bacterial metabolism. Comput Appl Biosci. 1986 Apr;2(1):23–27. doi: 10.1093/bioinformatics/2.1.23. [DOI] [PubMed] [Google Scholar]


