Abstract
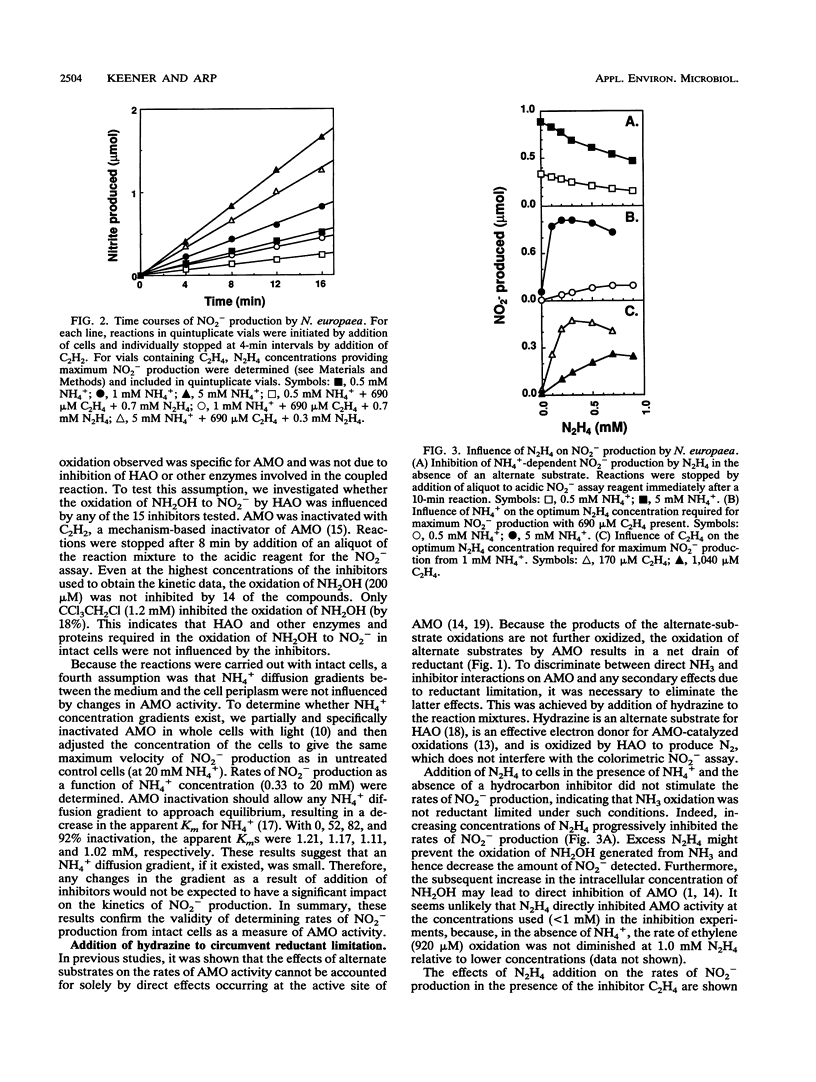
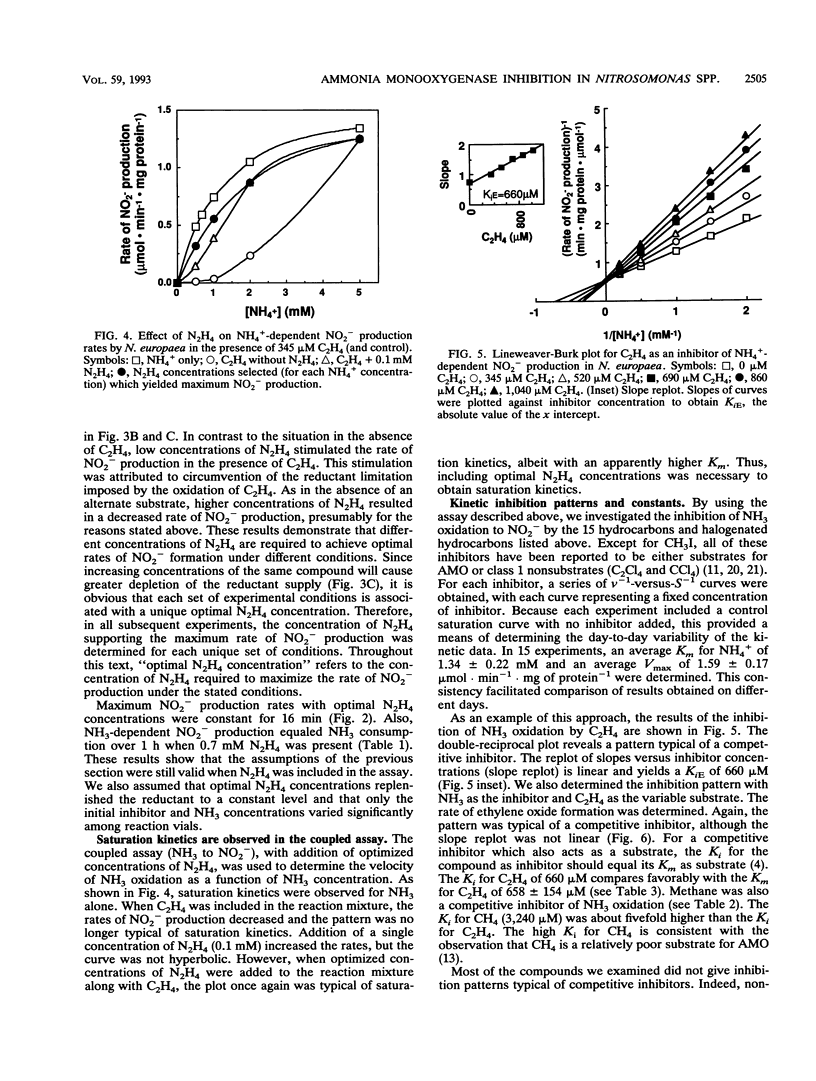
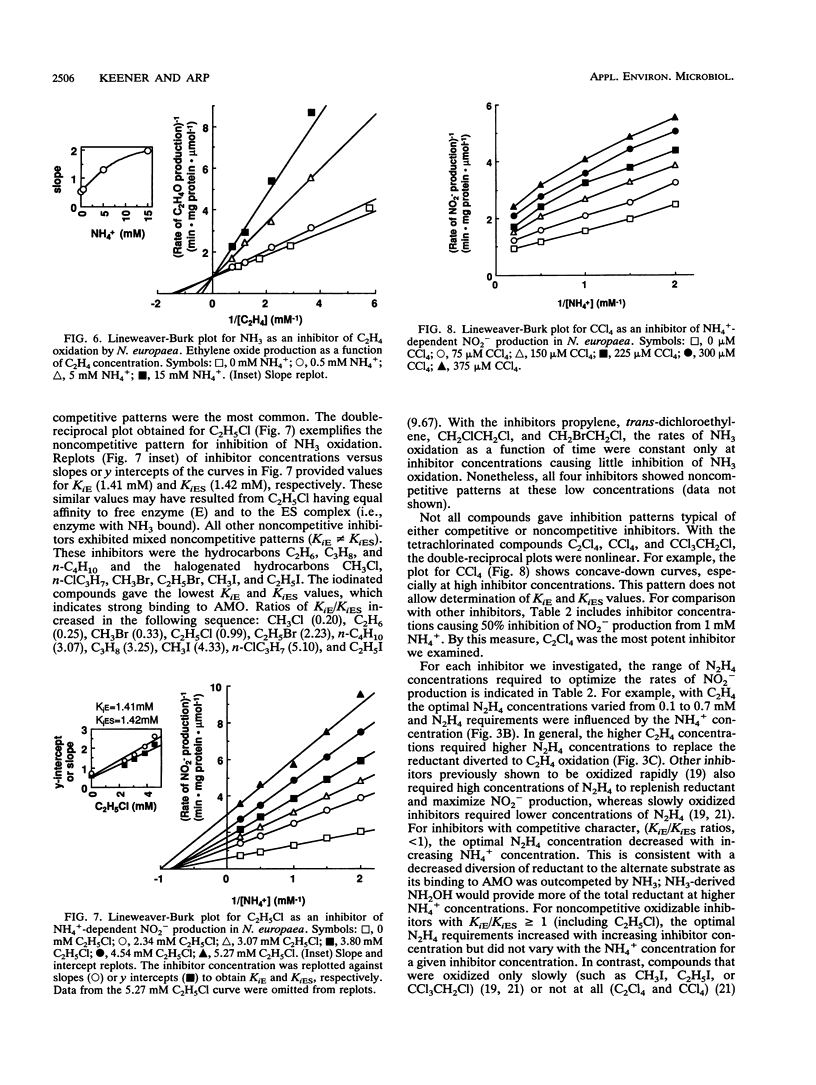
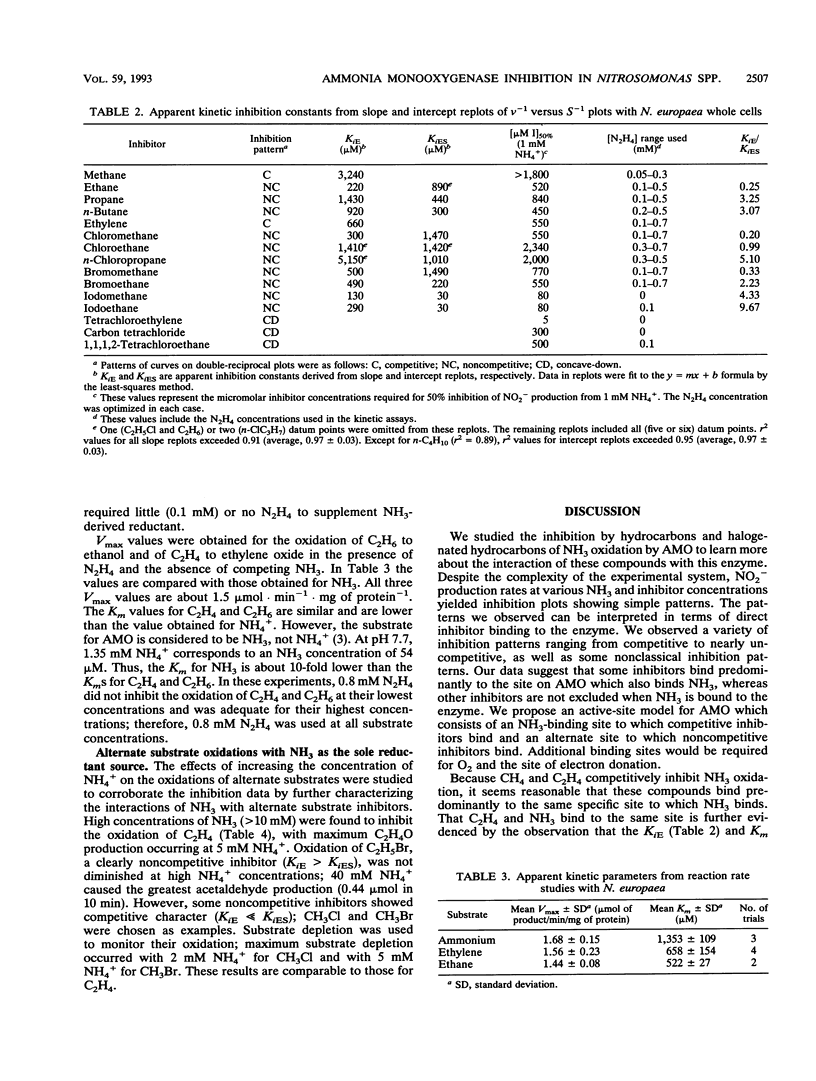
The inhibitory effects of 15 hydrocarbons and halogenated hydrocarbons on NH3 oxidation by ammonia monooxygenase (AMO) in intact cells of the nitrifying bacterium Nitrosomonas europaea were determined. Determination of AMO activity, measured as NO2- production, required coupling of hydroxylamine oxidoreductase (HAO) activity with NH3-dependent NH2OH production by AMO. Hydrazine, an alternate substrate for HAO, was added to the reaction mixtures as a source of reductant for AMO. Most inhibitors exhibited competitive or noncompetitive inhibition patterns. The competitive character generally decreased (KiE/KiES increased) as the molecular size of the inhibitors increased. For example, CH4 and C2H4 were competitive inhibitors of NH3 oxidation, whereas the remaining alkanes (up to C4) and monohalogenated (Cl, Br, I) alkanes were noncompetitive. Oxidation of C2H5Br (noncompetitive) increased as the NH4+ concentration increased up to 40 mM, whereas oxidations of inhibitors with competitive character (KiE ≪ KiES) were diminished at 40 mM NH4+. Multichlorinated compounds produced nonlinear Lineweaver-Burk plots. Iodinated alkanes (CH3I, C2H5I) and C2Cl4 were potent inhibitors of NH3 oxidation. Maximum rates of NH3, C2H4, and C2H6 oxidations were approximately equivalent, suggesting a common rate-determining step. These data support an active-site model for AMO consisting of an NH3-binding site and a second site that binds noncompetitive inhibitors, with oxidation occurring at either site.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON J. H. STUDIES ON THE OXIDATION OF AMMONIA BY NITROSOMONAS. Biochem J. 1965 Jun;95:688–698. doi: 10.1042/bj0950688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arciero D., Vannelli T., Logan M., Hooper A. B. Degradation of trichloroethylene by the ammonia-oxidizing bacterium Nitrosomonas europaea. Biochem Biophys Res Commun. 1989 Mar 15;159(2):640–643. doi: 10.1016/0006-291x(89)90042-9. [DOI] [PubMed] [Google Scholar]
- Bédard C., Knowles R. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol Rev. 1989 Mar;53(1):68–84. doi: 10.1128/mr.53.1.68-84.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign S. A., Hyman M. R., Arp D. J. In vitro activation of ammonia monooxygenase from Nitrosomonas europaea by copper. J Bacteriol. 1993 Apr;175(7):1971–1980. doi: 10.1128/jb.175.7.1971-1980.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Arp D. J. 14C2H2- and 14CO2-labeling studies of the de novo synthesis of polypeptides by Nitrosomonas europaea during recovery from acetylene and light inactivation of ammonia monooxygenase. J Biol Chem. 1992 Jan 25;267(3):1534–1545. [PubMed] [Google Scholar]
- Hyman M. R., Murton I. B., Arp D. J. Interaction of Ammonia Monooxygenase from Nitrosomonas europaea with Alkanes, Alkenes, and Alkynes. Appl Environ Microbiol. 1988 Dec;54(12):3187–3190. doi: 10.1128/aem.54.12.3187-3190.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Methane oxidation by Nitrosomonas europaea. Biochem J. 1983 Apr 15;212(1):31–37. doi: 10.1042/bj2120031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman M. R., Wood P. M. Suicidal inactivation and labelling of ammonia mono-oxygenase by acetylene. Biochem J. 1985 May 1;227(3):719–725. doi: 10.1042/bj2270719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEES H. The biochemistry of the nitrifying organisms. I. The ammonia oxidizing systems of Nitrosomonas. Biochem J. 1952 Sep;52(1):134–139. doi: 10.1042/bj0520134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M. B., Schendel F. J., Flickinger M. C., Nelsestuen G. L. Kinetic properties of enzyme populations in vivo: alkaline phosphatase of the Escherichia coli periplasm. Biochemistry. 1992 Nov 24;31(46):11500–11509. doi: 10.1021/bi00161a031. [DOI] [PubMed] [Google Scholar]
- Rasche M. E., Hicks R. E., Hyman M. R., Arp D. J. Oxidation of monohalogenated ethanes and n-chlorinated alkanes by whole cells of Nitrosomonas europaea. J Bacteriol. 1990 Sep;172(9):5368–5373. doi: 10.1128/jb.172.9.5368-5373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasche M. E., Hyman M. R., Arp D. J. Factors Limiting Aliphatic Chlorocarbon Degradation by Nitrosomonas europaea: Cometabolic Inactivation of Ammonia Monooxygenase and Substrate Specificity. Appl Environ Microbiol. 1991 Oct;57(10):2986–2994. doi: 10.1128/aem.57.10.2986-2994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasche Madeline E., Hyman Michael R., Arp Daniel J. Biodegradation of Halogenated Hydrocarbon Fumigants by Nitrifying Bacteria. Appl Environ Microbiol. 1990 Aug;56(8):2568–2571. doi: 10.1128/aem.56.8.2568-2571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki I., Kwok S. C., Dular U. Competitive inhibition of ammonia oxidation in Nitrosomonas europaea by methane, carbon monoxide or methanol. FEBS Lett. 1976 Dec 15;72(1):117–120. doi: 10.1016/0014-5793(76)80825-3. [DOI] [PubMed] [Google Scholar]
- Vannelli T., Logan M., Arciero D. M., Hooper A. B. Degradation of halogenated aliphatic compounds by the ammonia- oxidizing bacterium Nitrosomonas europaea. Appl Environ Microbiol. 1990 Apr;56(4):1169–1171. doi: 10.1128/aem.56.4.1169-1171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


