Abstract
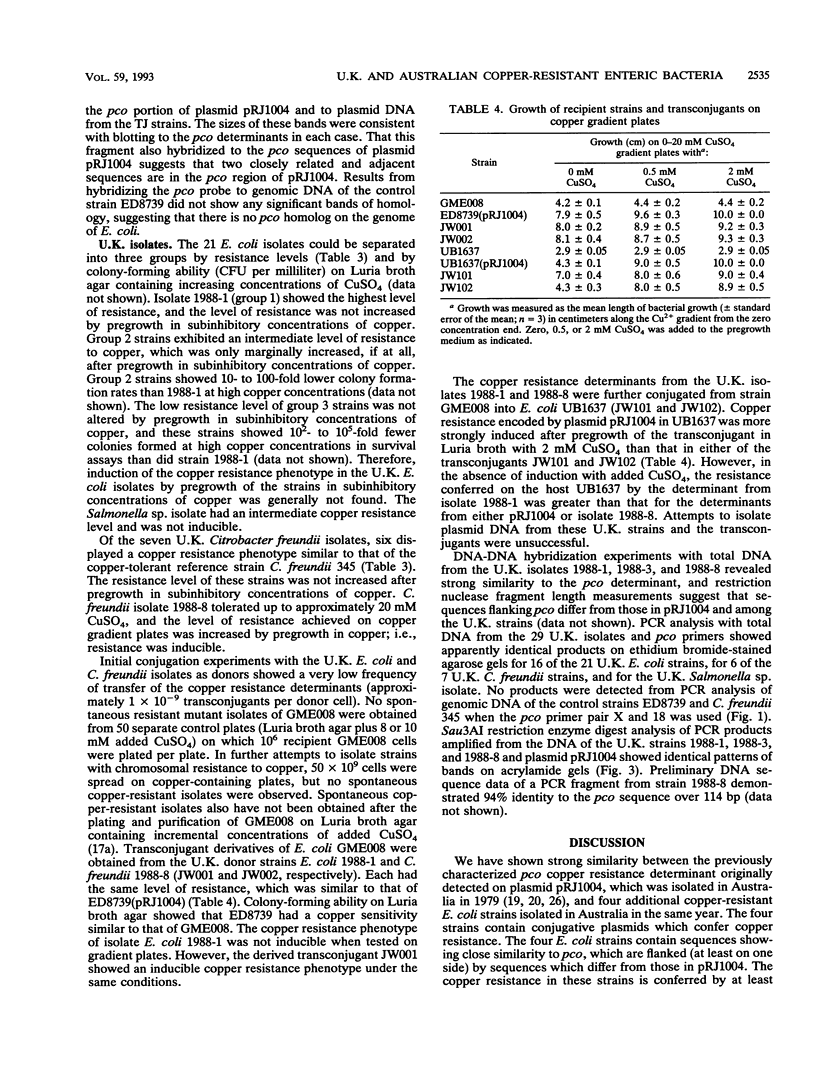
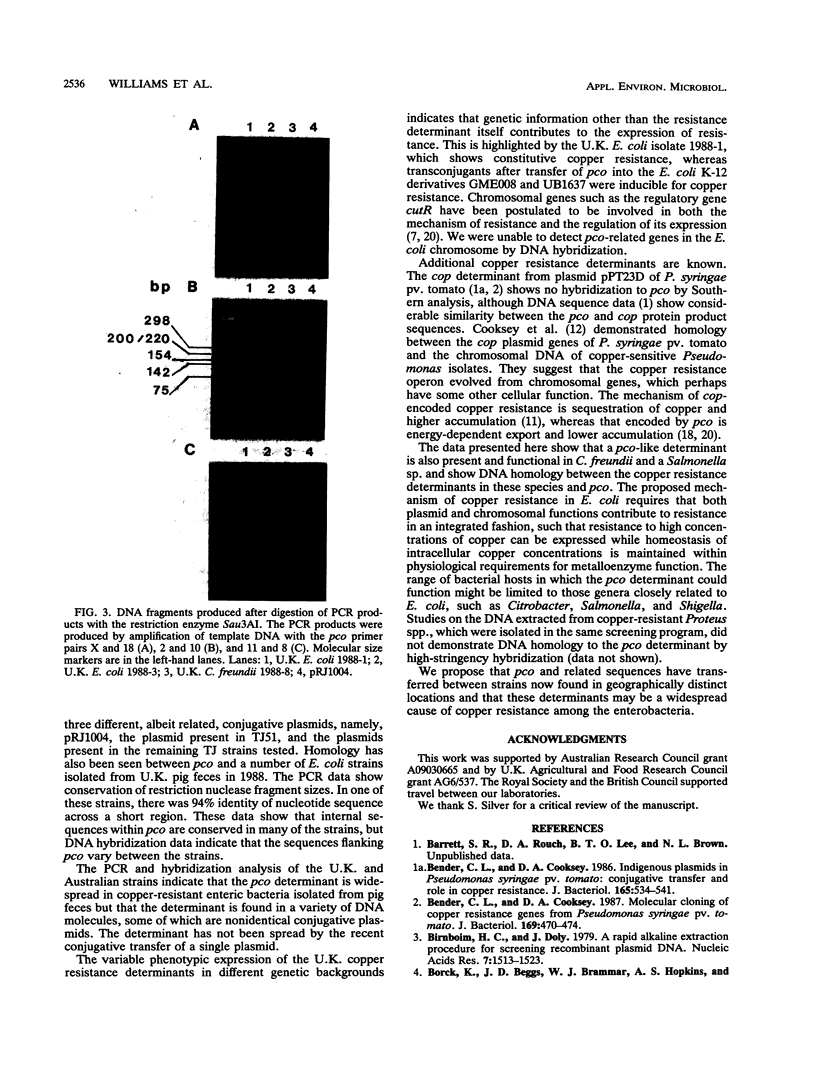
Thirty-three enteric isolates from Australian (Escherichia coli only) and United Kingdom (U.K.) (Salmonella sp., Citrobacter spp., and E. coli) piggeries were characterized with respect to their copper resistance. The copper resistance phenotypes of four new Australian E. coli isolates were comparable with that of the previously studied E. coli K-12 strain ED8739(pRJ1004), in that the resistance level in rich media was high (up to 18 mM CuSO4) and resistance was inducible. Copper resistance was transferable by conjugation from the new Australian isolates to E. coli K-12 recipients. DNA similarity between the new Australian isolates and the pco copper resistance determinant located on plasmid pRJ1004 was strong as measured by DNA-DNA hybridization; however, the copper resistance plasmids were nonidentical as indicated by the presence of restriction fragment length polymorphisms between the plasmids. DNA-DNA hybridization and polymerase chain reaction analysis demonstrated DNA homology between the pco determinant and DNA from the U.K.E. coli, Salmonella sp., and Citrobacter freundii isolates. However, the copper resistance level and inducibility were variable among the U.K. strains. Of the U.K. E. coli isolates, 1 demonstrated a high level of copper resistance, 4 exhibited intermediate resistance, and 16 showed a low level of copper resistance; all of these resistances were expressed constitutively. A single U.K. C. freundii isolate, had a high level of copper resistance, inducible by subtoxic levels of copper. Transconjugants from one E. coli and one C. freundii donor, with E. coli K-12 strain UB1637 as a recipient, showed copper resistance levels and inducibility of resistance which differed from that expressed from plasmid pRJ1004.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender C. L., Cooksey D. A. Indigenous plasmids in Pseudomonas syringae pv. tomato: conjugative transfer and role in copper resistance. J Bacteriol. 1986 Feb;165(2):534–541. doi: 10.1128/jb.165.2.534-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender C. L., Cooksey D. A. Molecular cloning of copper resistance genes from Pseudomonas syringae pv. tomato. J Bacteriol. 1987 Feb;169(2):470–474. doi: 10.1128/jb.169.2.470-474.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Brown N. L., Rouch D. A., Lee B. T. Copper resistance determinants in bacteria. Plasmid. 1992 Jan;27(1):41–51. doi: 10.1016/0147-619x(92)90005-u. [DOI] [PubMed] [Google Scholar]
- Cooksey D. A., Azad H. R., Cha J. S., Lim C. K. Copper resistance gene homologs in pathogenic and saprophytic bacterial species from tomato. Appl Environ Microbiol. 1990 Feb;56(2):431–435. doi: 10.1128/aem.56.2.431-435.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey D. A. Copper uptake and resistance in bacteria. Mol Microbiol. 1993 Jan;7(1):1–5. doi: 10.1111/j.1365-2958.1993.tb01091.x. [DOI] [PubMed] [Google Scholar]
- Edmonds M. S., Izquierdo O. A., Baker D. H. Feed additive studies with newly weaned pigs: efficacy of supplemental copper, antibiotics and organic acids. J Anim Sci. 1985 Feb;60(2):462–469. doi: 10.2527/jas1985.602462x. [DOI] [PubMed] [Google Scholar]
- Elek S. D., Higney L. Resistogram typing--a new epidemiological tool: application to Escherichia coli. J Med Microbiol. 1970 Feb;3(1):103–110. doi: 10.1099/00222615-3-1-103. [DOI] [PubMed] [Google Scholar]
- Hughes V. M., Datta N. Conjugative plasmids in bacteria of the 'pre-antibiotic' era. Nature. 1983 Apr 21;302(5910):725–726. doi: 10.1038/302725a0. [DOI] [PubMed] [Google Scholar]
- Rouch D., Camakaris J., Lee B. T., Luke R. K. Inducible plasmid-mediated copper resistance in Escherichia coli. J Gen Microbiol. 1985 Apr;131(4):939–943. doi: 10.1099/00221287-131-4-939. [DOI] [PubMed] [Google Scholar]
- Shurson G. C., Ku P. K., Waxler G. L., Yokoyama M. T., Miller E. R. Physiological relationships between microbiological status and dietary copper levels in the pig. J Anim Sci. 1990 Apr;68(4):1061–1071. doi: 10.2527/1990.6841061x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stansbury W. F., Tribble L. F., Orr D. E., Jr Effect of chelated copper sources on performance of nursery and growing pigs. J Anim Sci. 1990 May;68(5):1318–1322. doi: 10.2527/1990.6851318x. [DOI] [PubMed] [Google Scholar]
- Tetaz T. J., Luke R. K. Plasmid-controlled resistance to copper in Escherichia coli. J Bacteriol. 1983 Jun;154(3):1263–1268. doi: 10.1128/jb.154.3.1263-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Cruz F., Grinsted J. Genetic and molecular characterization of Tn21, a multiple resistance transposon from R100.1. J Bacteriol. 1982 Jul;151(1):222–228. doi: 10.1128/jb.151.1.222-228.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]