Abstract
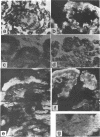
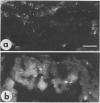
A thermophilic upflow anaerobic sludge blanket (UASB) reactor degrading acetate was started by applying published methods (W. M. Wiegant and A. W. A. de Man, Biotechnol. Bioeng. 28:718-77, 1986) for production of granules dominated by Methanothrix spp. The reactor was inoculated with thermophilic digested sludge. No granules were observed during the first 7 months of start-up of the UASB reactor. However, after the concentrations of potassium, phosphate, ammonium, and magnesium in the medium were gradually increased, granules developed, indicating that there was a critical concentration of one or more of the ions required for production of granules from the starting material. After several years of stable operation, the effect of removing 60% of the granular sludge was investigated. Immunologic qualitative and quantitative studies showed that removal of the granular sludge resulted in an increase in the number of the predominant methanogens, antigenically related to Methanosarcina thermophila TM-1 and Methanosarcina mazeii S-6, and Methanobacterium thermoautotrophicum delta H and GC1. These changes were accompanied by modifications of the microanatomy of the granules, as demonstrated histochemically and immunohistochemically. The results indicated that different catabolic pathways dominated in different regions of the granules, i.e., acetate oxidation in the middle of the granules, where there is a low acetate concentration, and an aceticlastic reaction in the outer surfaces, with a high acetate concentration. The results also showed that removal of granules from a UASB reactor which has been under steady-state operation for a long period can improve the reactor's performance via formation of denser and larger granules with improved microbial activities.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Mah R. A. Effects of Calcium, Magnesium, pH, and Extent of Growth on the Morphology of Methanosarcina mazei S-6. Appl Environ Microbiol. 1987 Jul;53(7):1699–1700. doi: 10.1128/aem.53.7.1699-1700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotenhuis J. T., Smit M., Plugge C. M., Xu Y. S., van Lammeren A. A., Stams A. J., Zehnder A. J. Bacteriological composition and structure of granular sludge adapted to different substrates. Appl Environ Microbiol. 1991 Jul;57(7):1942–1949. doi: 10.1128/aem.57.7.1942-1949.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. J., Zinder S. H. Hydrogen partial pressures in a thermophilic acetate-oxidizing methanogenic coculture. Appl Environ Microbiol. 1988 Jun;54(6):1457–1461. doi: 10.1128/aem.54.6.1457-1461.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macario A. J., Conway de Macario E. Quantitative immunologic analysis of the methanogenic flora of digestors reveals a considerable diversity. Appl Environ Microbiol. 1988 Jan;54(1):79–86. doi: 10.1128/aem.54.1.79-86.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayerhofer L. E., Macario A. J., Conway de Macario E. Lamina, a novel multicellular form of Methanosarcina mazei S-6. J Bacteriol. 1992 Jan;174(1):309–314. doi: 10.1128/jb.174.1.309-314.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min H., Zinder S. H. Kinetics of Acetate Utilization by Two Thermophilic Acetotrophic Methanogens: Methanosarcina sp. Strain CALS-1 and Methanothrix sp. Strain CALS-1. Appl Environ Microbiol. 1989 Feb;55(2):488–491. doi: 10.1128/aem.55.2.488-491.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R. W., Akin D. E., Nordstedt R. A., Thomas M. V., Aldrich H. C. Light and electron microscopic examinations of methane-producing biofilms from anaerobic fixed-bed reactors. Appl Environ Microbiol. 1984 Jul;48(1):127–136. doi: 10.1128/aem.48.1.127-136.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. E., Macario A. J., Ahring B. K., Conway de Macario E. Effect of magnesium on methanogenic subpopulations in a thermophilic acetate-degrading granular consortium. Appl Environ Microbiol. 1992 Mar;58(3):862–868. doi: 10.1128/aem.58.3.862-868.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. H., Mah R. A. Kinetics of acetate metabolism during sludge digestion. Appl Microbiol. 1966 May;14(3):368–371. doi: 10.1128/am.14.3.368-371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser F. A., van Lier J. B., Macario A. J., Conway de Macario E. Diversity and population dynamics of methanogenic bacteria in a granular consortium. Appl Environ Microbiol. 1991 Jun;57(6):1728–1734. doi: 10.1128/aem.57.6.1728-1734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann P., Ahring B. K., Mah R. A. Threshold acetate concentrations for acetate catabolism by aceticlastic methanogenic bacteria. Appl Environ Microbiol. 1989 Feb;55(2):514–515. doi: 10.1128/aem.55.2.514-515.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun L., Boone D. R., Mah R. A. Control of the Life Cycle of Methanosarcina mazei S-6 by Manipulation of Growth Conditions. Appl Environ Microbiol. 1988 Aug;54(8):2064–2068. doi: 10.1128/aem.54.8.2064-2068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Anguish T., Cardwell S. C. Effects of Temperature on Methanogenesis in a Thermophilic (58 degrees C) Anaerobic Digestor. Appl Environ Microbiol. 1984 Apr;47(4):808–813. doi: 10.1128/aem.47.4.808-813.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Anguish T., Cardwell S. C. Selective inhibition by 2-bromoethanesulfonate of methanogenesis from acetate in a thermophilic anaerobic digestor. Appl Environ Microbiol. 1984 Jun;47(6):1343–1345. doi: 10.1128/aem.47.6.1343-1345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Mah R. A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H(2)-CO(2) for Methanogenesis. Appl Environ Microbiol. 1979 Nov;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]