Abstract
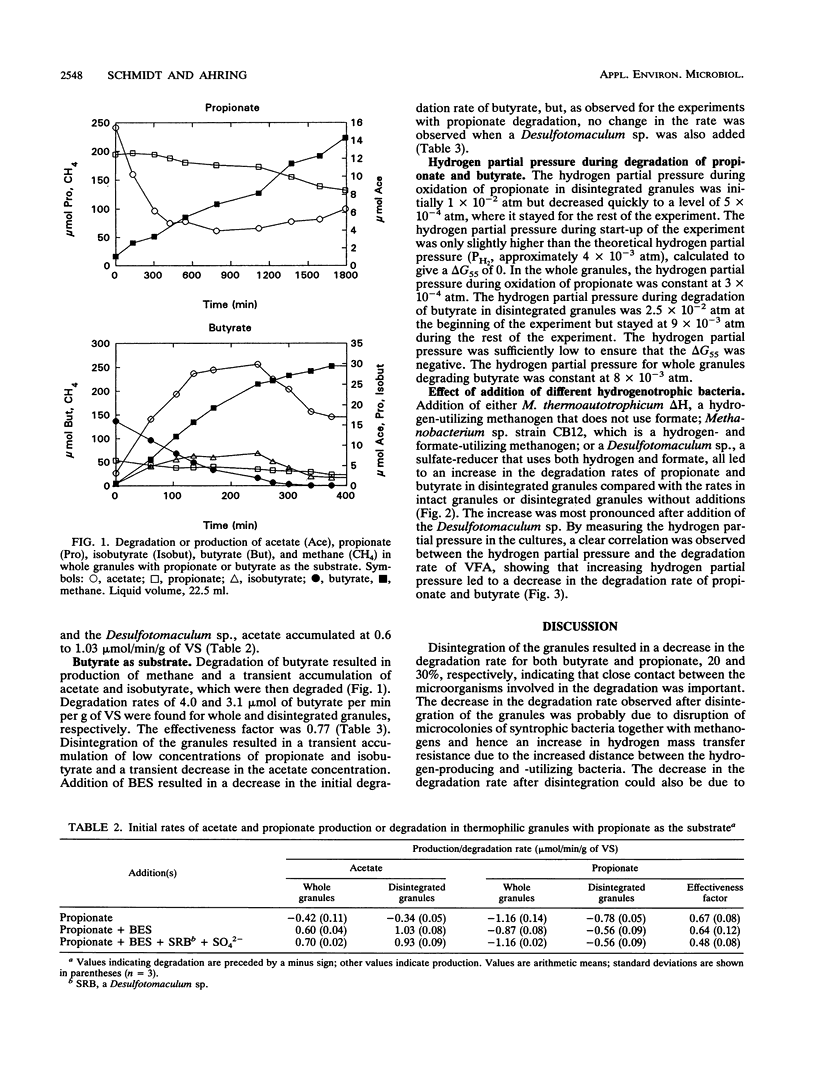
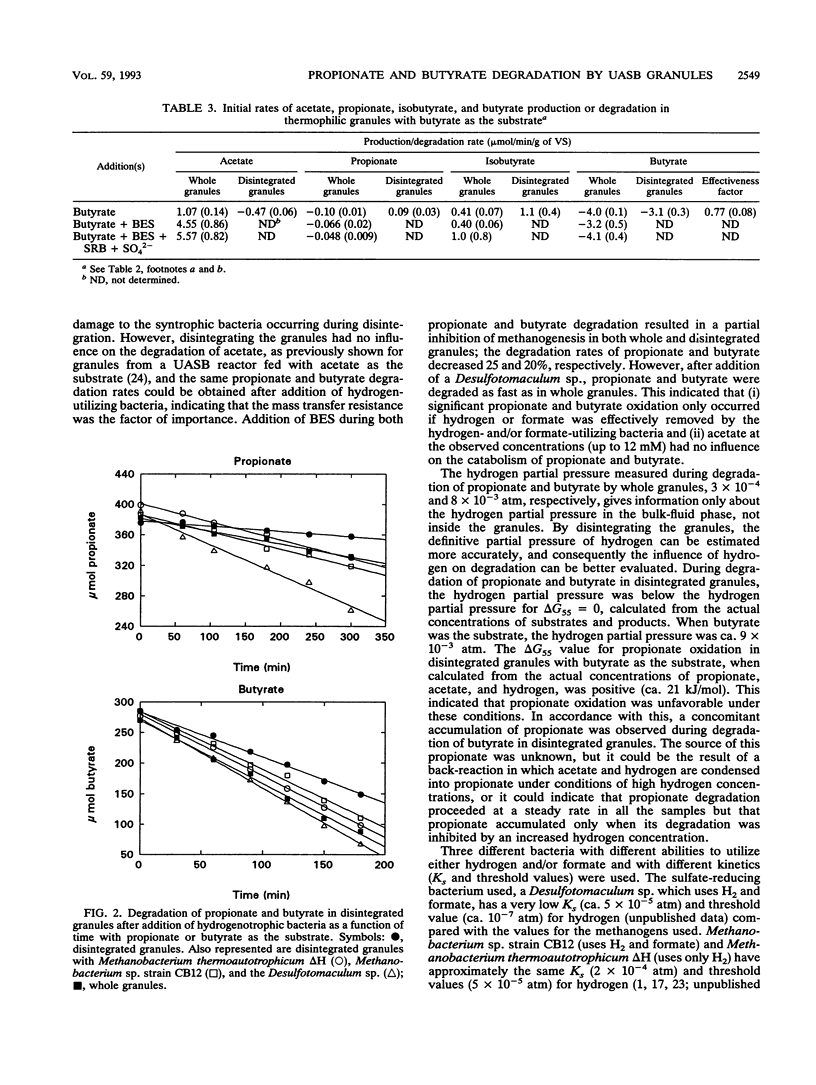
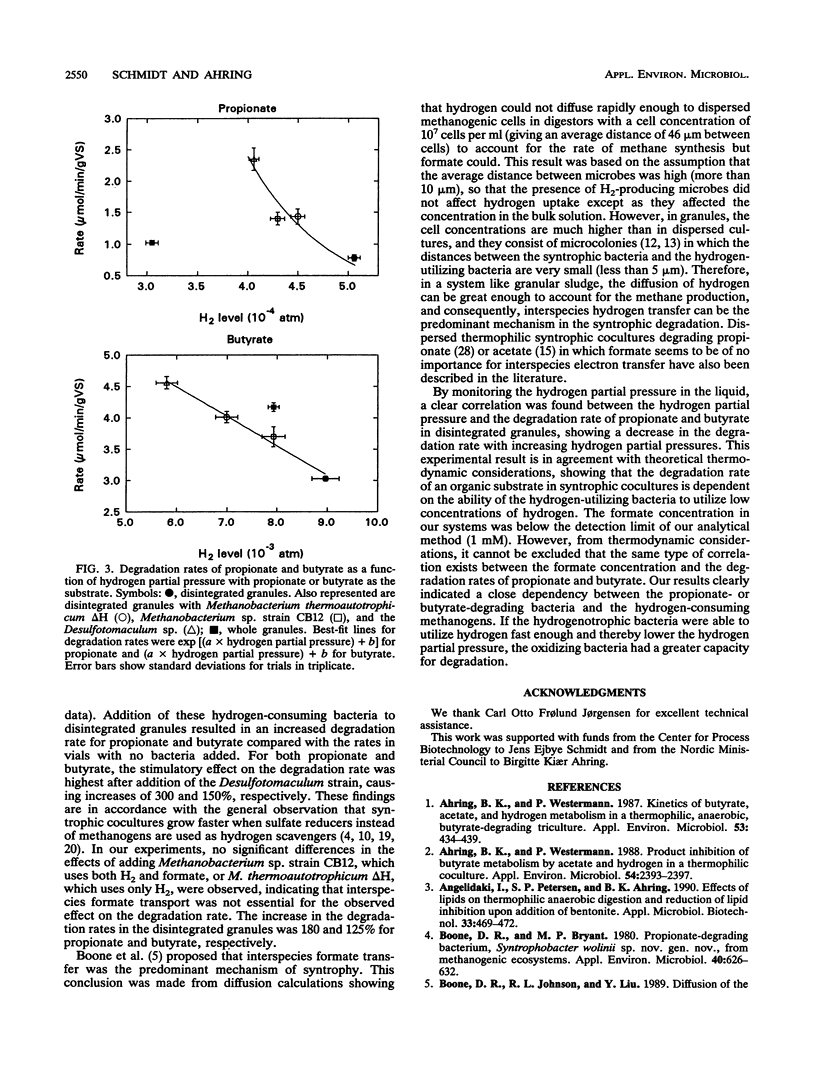
Degradation of propionate and butyrate in whole and disintegrated granules from a thermophilic (55 degrees C) upflow anaerobic sludge blanket reactor fed with acetate, propionate, and butyrate as substrates was examined. The propionate and butyrate degradation rates in whole granules were 1.16 and 4.0 mumol/min/g of volatile solids, respectively, and the rates decreased 35 and 25%, respectively, after disintegration of the granules. The effect of adding different hydrogen-oxidizing bacteria (both sulfate reducers and methanogens), some of which used formate in addition to hydrogen, to disintegrated granules was tested. Addition of either Methanobacterium thermoautotrophicum delta H, a hydrogen-utilizing methanogen that does not use formate, or Methanobacterium sp. strain CB12, a hydrogen- and formate-utilizing methanogen, to disintegrated granules increased the degradation rate of both propionate and butyrate. Furthermore, addition of a thermophilic sulfate-reducing bacterium (a Desulfotomaculum sp. isolated in our laboratory) to disintegrated granules improved the degradation of both substrates even more than the addition of methanogens. By monitoring the hydrogen partial pressure in the cultures, a correlation between the hydrogen partial pressure and the degradation rate of propionate and butyrate was observed, showing a decrease in the degradation rate with increased hydrogen partial pressure. No significant differences in the stimulation of the degradation rates were observed when the disintegrated granules were supplied with methanogens that utilized hydrogen only or hydrogen and formate. This indicated that interspecies formate transfer was not important for stimulation of propionate and butyrate degradation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahring B. K., Westermann P. Kinetics of butyrate, acetate, and hydrogen metabolism in a thermophilic, anaerobic, butyrate-degrading triculture. Appl Environ Microbiol. 1987 Feb;53(2):434–439. doi: 10.1128/aem.53.2.434-439.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahring B. K., Westermann P. Product inhibition of butyrate metabolism by acetate and hydrogen in a thermophilic coculture. Appl Environ Microbiol. 1988 Oct;54(10):2393–2397. doi: 10.1128/aem.54.10.2393-2397.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelidaki I., Petersen S. P., Ahring B. K. Effects of lipids on thermophilic anaerobic digestion and reduction of lipid inhibition upon addition of bentonite. Appl Microbiol Biotechnol. 1990 Jul;33(4):469–472. doi: 10.1007/BF00176668. [DOI] [PubMed] [Google Scholar]
- Boone D. R., Bryant M. P. Propionate-Degrading Bacterium, Syntrophobacter wolinii sp. nov. gen. nov., from Methanogenic Ecosystems. Appl Environ Microbiol. 1980 Sep;40(3):626–632. doi: 10.1128/aem.40.3.626-632.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer E. J., McCarty P. L. Effects of 2-bromoethanesulfonic Acid and 2- chloroethanesulfonic Acid on acetate utilization in a continuous-flow methanogenic fixed-film column. Appl Environ Microbiol. 1983 Apr;45(4):1408–1410. doi: 10.1128/aem.45.4.1408-1410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. P., Wolin E. A., Wolin M. J., Wolfe R. S. Methanobacillus omelianskii, a symbiotic association of two species of bacteria. Arch Mikrobiol. 1967;59(1):20–31. doi: 10.1007/BF00406313. [DOI] [PubMed] [Google Scholar]
- Conrad R., Phelps T. J., Zeikus J. G. Gas metabolism evidence in support of the juxtaposition of hydrogen-producing and methanogenic bacteria in sewage sludge and lake sediments. Appl Environ Microbiol. 1985 Sep;50(3):595–601. doi: 10.1128/aem.50.3.595-601.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. J., Zinder S. H. Hydrogen partial pressures in a thermophilic acetate-oxidizing methanogenic coculture. Appl Environ Microbiol. 1988 Jun;54(6):1457–1461. doi: 10.1128/aem.54.6.1457-1461.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R. Minimum threshold for hydrogen metabolism in methanogenic bacteria. Appl Environ Microbiol. 1985 Jun;49(6):1530–1531. doi: 10.1128/aem.49.6.1530-1531.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McInerney M. J., Bryant M. P., Hespell R. B., Costerton J. W. Syntrophomonas wolfei gen. nov. sp. nov., an Anaerobic, Syntrophic, Fatty Acid-Oxidizing Bacterium. Appl Environ Microbiol. 1981 Apr;41(4):1029–1039. doi: 10.1128/aem.41.4.1029-1039.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauss A., Samson R., Guiot S. Thermodynamic evidence of trophic microniches in methanogenic granular sludge-bed reactors. Appl Microbiol Biotechnol. 1990 Apr;33(1):88–92. doi: 10.1007/BF00170577. [DOI] [PubMed] [Google Scholar]
- Phelps T. J., Conrad R., Zeikus J. G. Sulfate-Dependent Interspecies H(2) Transfer between Methanosarcina barkeri and Desulfovibrio vulgaris during Coculture Metabolism of Acetate or Methanol. Appl Environ Microbiol. 1985 Sep;50(3):589–594. doi: 10.1128/aem.50.3.589-594.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. E., Macario A. J., Ahring B. K., Conway de Macario E. Effect of magnesium on methanogenic subpopulations in a thermophilic acetate-degrading granular consortium. Appl Environ Microbiol. 1992 Mar;58(3):862–868. doi: 10.1128/aem.58.3.862-868.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. H., Mah R. A. Kinetics of acetate metabolism during sludge digestion. Appl Microbiol. 1966 May;14(3):368–371. doi: 10.1128/am.14.3.368-371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stams A. J., Grolle K. C., Frijters C. T., Van Lier J. B. Enrichment of Thermophilic Propionate-Oxidizing Bacteria in Syntrophy with Methanobacterium thermoautotrophicum or Methanobacterium thermoformicicum. Appl Environ Microbiol. 1992 Jan;58(1):346–352. doi: 10.1128/aem.58.1.346-352.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele Jurgen H., Chartrain M., Zeikus J. Gregory. Control of Interspecies Electron Flow during Anaerobic Digestion: Role of Floc Formation in Syntrophic Methanogenesis. Appl Environ Microbiol. 1988 Jan;54(1):10–19. doi: 10.1128/aem.54.1.10-19.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann P., Ahring B. K., Mah R. A. Temperature Compensation in Methanosarcina barkeri by Modulation of Hydrogen and Acetate Affinity. Appl Environ Microbiol. 1989 May;55(5):1262–1266. doi: 10.1128/aem.55.5.1262-1266.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhang H., Boone D. R., Mah R. A. Isolation and characterization of a fast-growing, thermophilic methanobacterium species. Appl Environ Microbiol. 1986 Nov;52(5):1227–1229. doi: 10.1128/aem.52.5.1227-1229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinder S. H., Anguish T., Cardwell S. C. Selective inhibition by 2-bromoethanesulfonate of methanogenesis from acetate in a thermophilic anaerobic digestor. Appl Environ Microbiol. 1984 Jun;47(6):1343–1345. doi: 10.1128/aem.47.6.1343-1345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


