Abstract
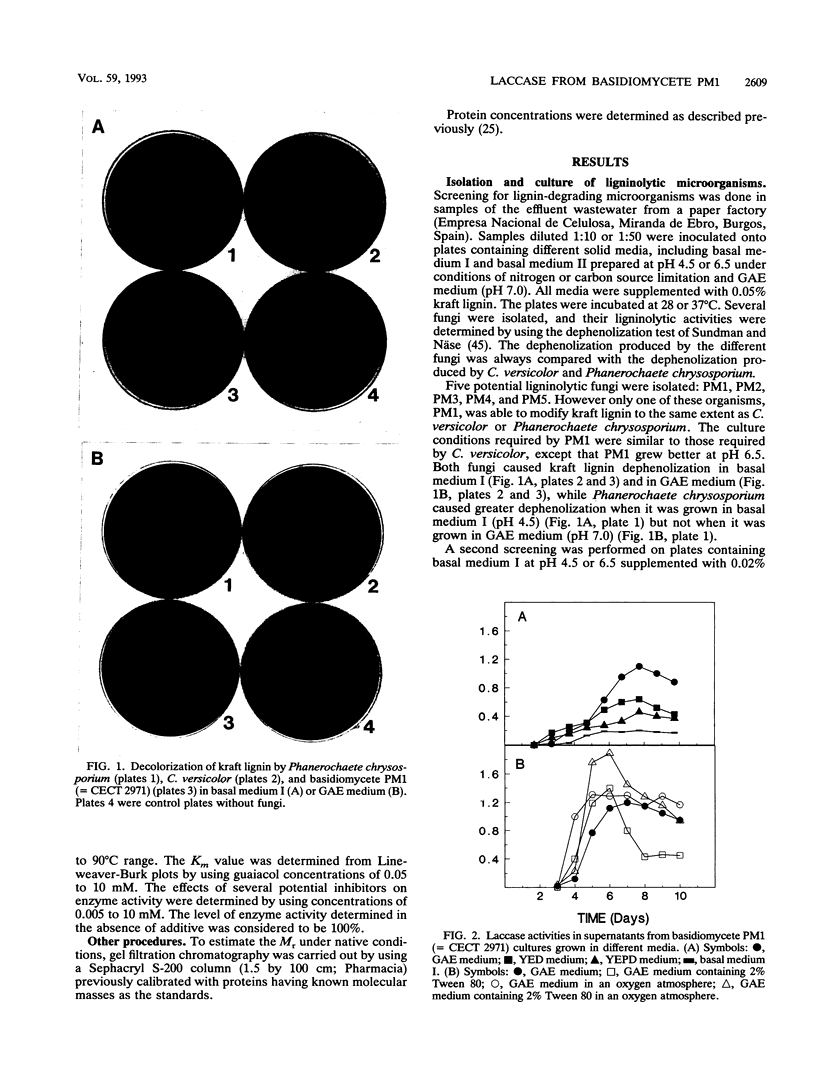
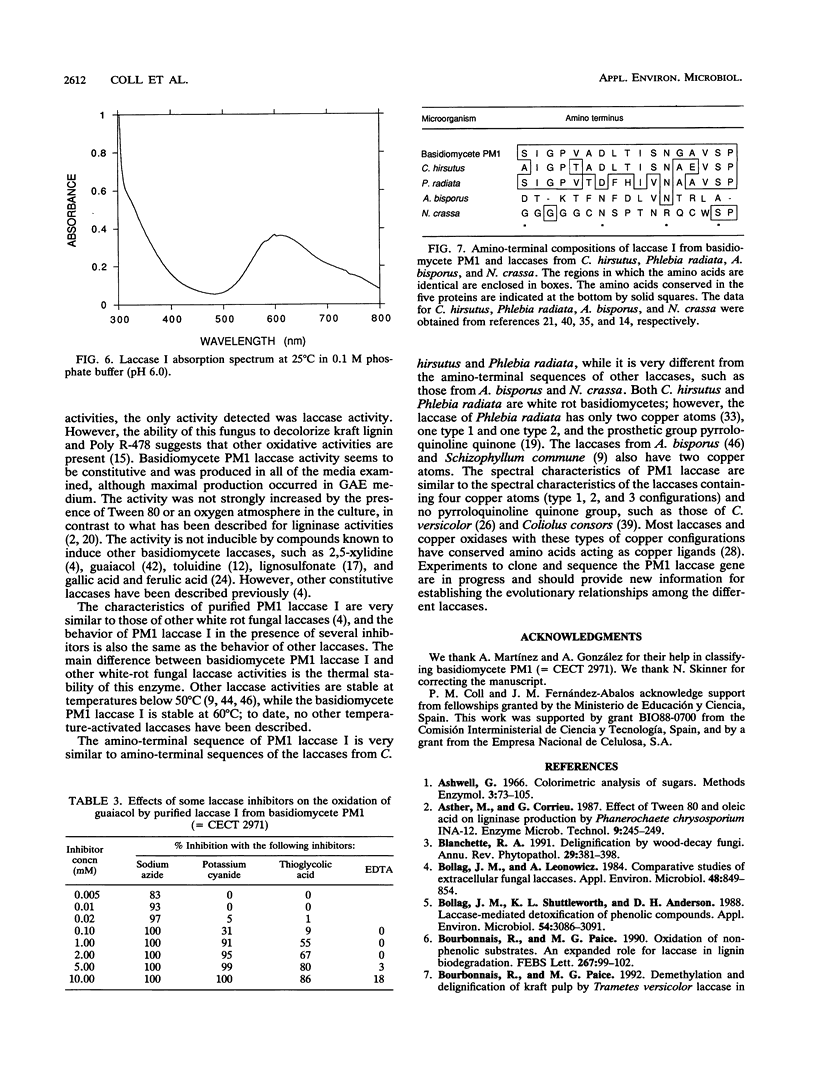
A new lignin-degrading basidiomycete, strain PM1 (= CECT 2971), was isolated from the wastewater of a paper factory. The major ligninolytic activity detected in the basidiomycete PM1 culture supernatant was a phenoloxidase (laccase). This activity was produced constitutively in defined or complex media and appeared as two protein bands in native gel electrophoresis preparations. No enzyme induction was found after treatment with certain potential laccase inducers. Laccase I was purified to homogeneity by gel filtration chromatography, anion-exchange chromatography, and hydrophobicity chromatography. The enzyme is a monomeric glycoprotein containing 6.5% carbohydrate and having a molecular weight of 64,000. It has an isoelectric point of 3.6, it is stable in a pH range from 3 to 9, and its optimum pH is 4.5. The laccase optimal reaction temperature is 80 degrees C, the laccase is stable for 1 h at 60 degrees C, and its activity increases with temperature. Spectroscopic analysis revealed that the enzyme has four bound copper atoms, a type I copper, a type II copper, and a type III binuclear copper. The amino-terminal sequence of the protein is very similar to the amino-terminal sequences of laccases from Coriolus hirsutus and Phlebia radiata.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollag J. M., Leonowicz A. Comparative studies of extracellular fungal laccases. Appl Environ Microbiol. 1984 Oct;48(4):849–854. doi: 10.1128/aem.48.4.849-854.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollag J. M., Shuttleworth K. L., Anderson D. H. Laccase-mediated detoxification of phenolic compounds. Appl Environ Microbiol. 1988 Dec;54(12):3086–3091. doi: 10.1128/aem.54.12.3086-3091.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbonnais R., Paice M. G. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990 Jul 2;267(1):99–102. doi: 10.1016/0014-5793(90)80298-w. [DOI] [PubMed] [Google Scholar]
- Clutterbuck A. J. The genetics of conidiophore pigmentation in Aspergillus nidulans. J Gen Microbiol. 1990 Sep;136(9):1731–1738. doi: 10.1099/00221287-136-9-1731. [DOI] [PubMed] [Google Scholar]
- Fåhraeus G., Reinhammar B. Large scale production and purification of laccase from cultures of the fungus Polyporus versicolor and some properties of laccase A. Acta Chem Scand. 1967;21(9):2367–2378. doi: 10.3891/acta.chem.scand.21-2367. [DOI] [PubMed] [Google Scholar]
- Germann U. A., Müller G., Hunziker P. E., Lerch K. Characterization of two allelic forms of Neurospora crassa laccase. Amino- and carboxyl-terminal processing of a precursor. J Biol Chem. 1988 Jan 15;263(2):885–896. [PubMed] [Google Scholar]
- Karhunen E., Niku-Paavola M. L., Viikari L., Haltia T., van der Meer R. A., Duine J. A. A novel combination of prosthetic groups in a fungal laccase; PQQ and two copper atoms. FEBS Lett. 1990 Jul 2;267(1):6–8. doi: 10.1016/0014-5793(90)80273-l. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Kojima Y., Tsukuda Y., Kawai Y., Tsukamoto A., Sugiura J., Sakaino M., Kita Y. Cloning, sequence analysis, and expression of ligninolytic phenoloxidase genes of the white-rot basidiomycete Coriolus hirsutus. J Biol Chem. 1990 Sep 5;265(25):15224–15230. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leonowicz A., Trojanowski J. Induction of laccase by ferulic acid in basidiomycetes. Acta Biochim Pol. 1975;22(4):291–295. [PubMed] [Google Scholar]
- Maccarrone M., Veldink G. A., Vliegenthart J. F. An investigation on the quinoprotein nature of some fungal and plant oxidoreductases. J Biol Chem. 1991 Nov 5;266(31):21014–21017. [PubMed] [Google Scholar]
- Messerschmidt A., Huber R. The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships. Eur J Biochem. 1990 Jan 26;187(2):341–352. doi: 10.1111/j.1432-1033.1990.tb15311.x. [DOI] [PubMed] [Google Scholar]
- Michel F. C., Jr, Dass S. B., Grulke E. A., Reddy C. A. Role of manganese peroxidases and lignin peroxidases of Phanerochaete chrysosporium in the decolorization of kraft bleach plant effluent. Appl Environ Microbiol. 1991 Aug;57(8):2368–2375. doi: 10.1128/aem.57.8.2368-2375.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Niku-Paavola M. L., Karhunen E., Salola P., Raunio V. Ligninolytic enzymes of the white-rot fungus Phlebia radiata. Biochem J. 1988 Sep 15;254(3):877–883. doi: 10.1042/bj2540877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C. R., Matcham S. E., Wood D. A., Thurston C. F. The structure of laccase protein and its synthesis by the commercial mushroom Agaricus bisporus. J Gen Microbiol. 1993 Jan;139(1):171–178. doi: 10.1099/00221287-139-1-171. [DOI] [PubMed] [Google Scholar]
- Périé F. H., Gold M. H. Manganese regulation of manganese peroxidase expression and lignin degradation by the white rot fungus Dichomitus squalens. Appl Environ Microbiol. 1991 Aug;57(8):2240–2245. doi: 10.1128/aem.57.8.2240-2245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloheimo M., Niku-Paavola M. L., Knowles J. K. Isolation and structural analysis of the laccase gene from the lignin-degrading fungus Phlebia radiata. J Gen Microbiol. 1991 Jul;137(7):1537–1544. doi: 10.1099/00221287-137-7-1537. [DOI] [PubMed] [Google Scholar]
- Sannia G., Limongi P., Cocca E., Buonocore F., Nitti G., Giardina P. Purification and characterization of a veratryl alcohol oxidase enzyme from the lignin degrading basidiomycete Pleurotus ostreatus. Biochim Biophys Acta. 1991 Jan 23;1073(1):114–119. doi: 10.1016/0304-4165(91)90190-r. [DOI] [PubMed] [Google Scholar]





