Abstract
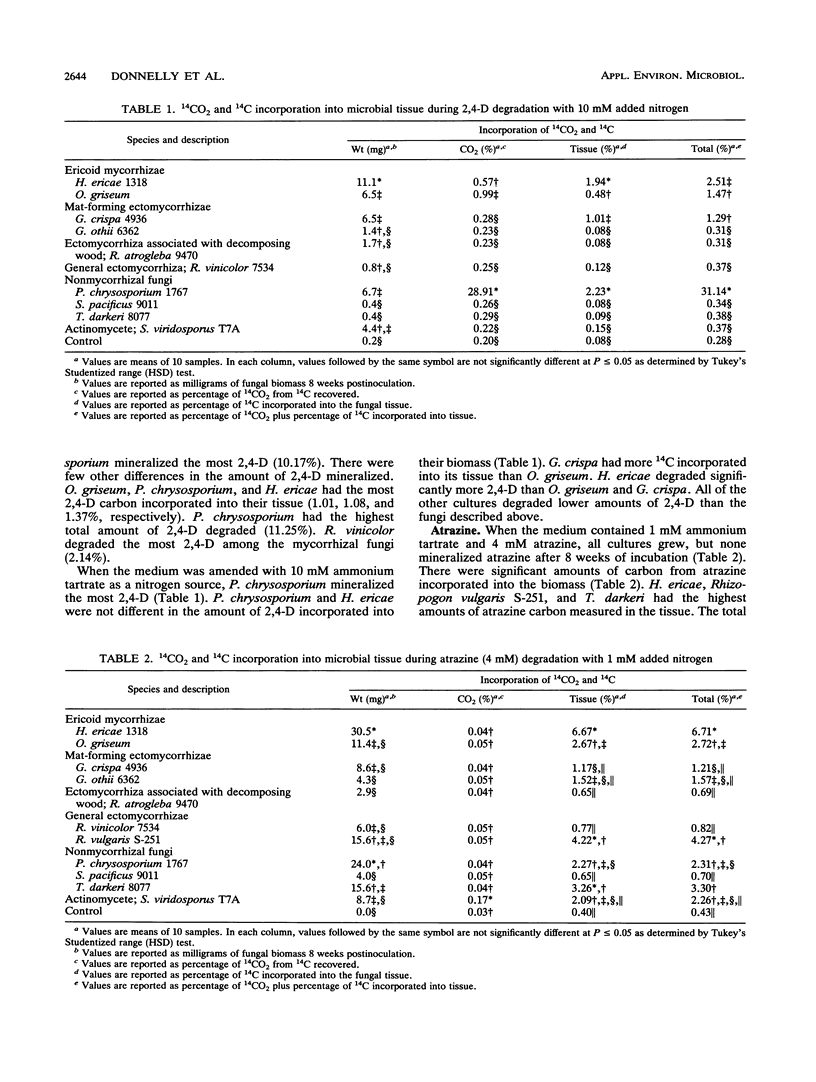
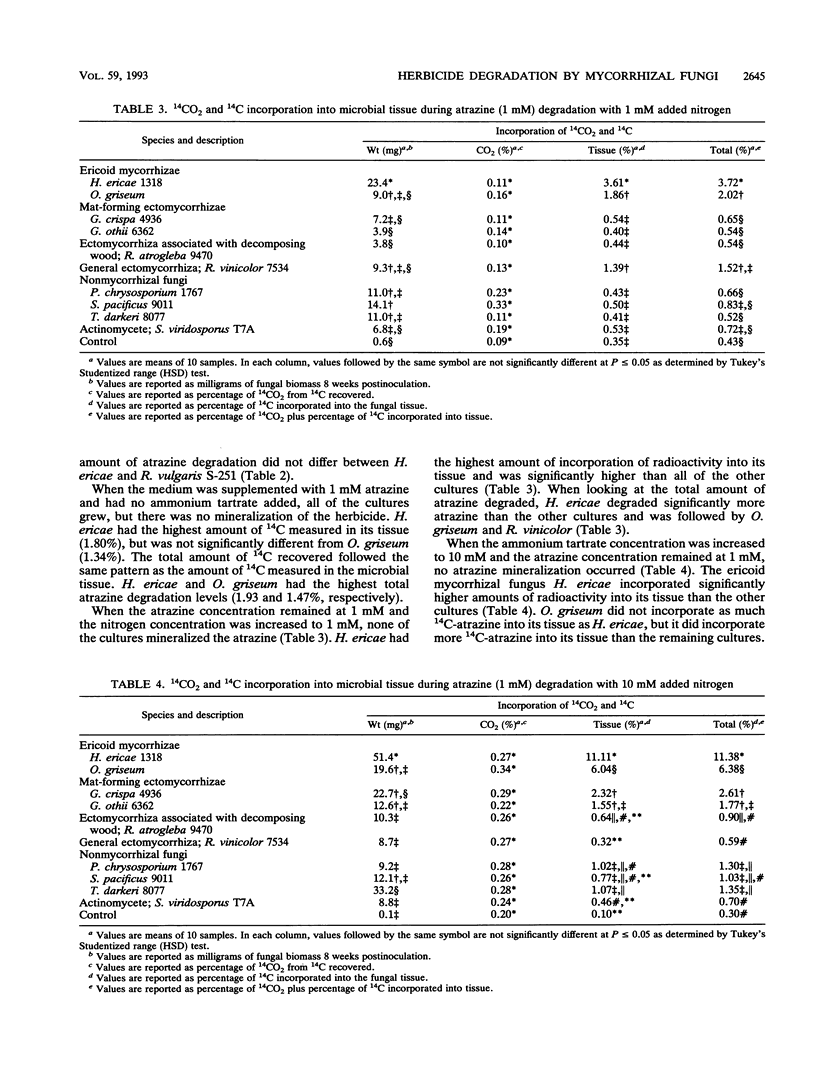
Nine mycorrhizal fungi and free-living saprophytic microorganisms were tested for their ability to degrade two chlorinated aromatic herbicides at two herbicide concentrations and three nitrogen concentrations. Radiolabelled 2,4-dichlorophenoxyacetic acid (2,4-D) and 2-chloro-4-ethylamino-6-isopropylamino-s-triazine (atrazine) were used as substrates at concentrations of 1 and 4 mM. After 8 weeks, none of the cultures tested grew at 4 mM 2,4-D. However, when the 2,4-D concentration was reduced to 1 mM, Phanerochaete chrysosporium 1767 had the highest level of 2,4-D mineralization and degradation under all nitrogen conditions. All cultures tested grew at both atrazine concentrations. In all cases, the ericoid mycorrhizal fungus Hymenoscyphus ericae 1318 had the highest level of atrazine carbon incorporated into its tissue. In general, as the nitrogen concentration increased, the total herbicide degradation increased. All of the cultures, except for Rhizopogon vinicolor 7534 and Sclerogaster pacificus 9011, showed increased degradation at 4 mM compared with 1 mM atrazine. The ability to degrade these two herbicides thus appeared to be dependent on the fungus and the herbicide, with no correlation to fungal ecotype (mycorrhizal versus free living).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bae K. S., Barton L. L. Alkaline Phosphatase and Other Hydrolyases Produced by Cenococcum graniforme, an Ectomycorrhizal Fungus. Appl Environ Microbiol. 1989 Oct;55(10):2511–2516. doi: 10.1128/aem.55.10.2511-2516.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry G. R., Chapalamadugu S. Biodegradation of halogenated organic compounds. Microbiol Rev. 1991 Mar;55(1):59–79. doi: 10.1128/mr.55.1.59-79.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. P., Hallberg K. B., Tuovinen O. H. Biological degradation of 2,4-dichlorophenoxyacetic acid: chloride mass balance in stirred tank reactors. Appl Environ Microbiol. 1989 Oct;55(10):2717–2719. doi: 10.1128/aem.55.10.2717-2719.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahasrabudhe S. R., Modi V. V. Microbial degradation of chlorinated aromatic compounds. Microbiol Sci. 1987 Oct;4(10):300–303. [PubMed] [Google Scholar]
- Zhu H., Guo D. C., Dancik B. P. Purification and Characterization of an Extracellular Acid Proteinase from the Ectomycorrhizal Fungus Hebeloma crustuliniforme. Appl Environ Microbiol. 1990 Apr;56(4):837–843. doi: 10.1128/aem.56.4.837-843.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


