Abstract
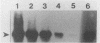
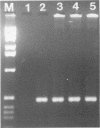
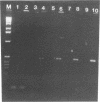
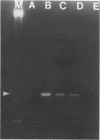
Hepatitis A virus (HAV) is a major cause of infectious hepatitis in humans. In this respect, bivalve mollusks pose a major health concern because they are filter feeders and can concentrate the virus up to 900-fold from contaminated water. Detection of HAV has been hampered because wild-type HAV grows poorly if at all in cell culture. Here we describe a technique for the detection of HAV in shellfish based on reverse transcription coupled with the polymerase chain reaction. RNA is isolated from hard-shell clam tissue and reverse transcribed with avian myeloblastosis virus reverse transcriptase. A portion of the cDNA pool is then amplified with primers specific for HAV. In experiments with an in vitro-synthesized HAV transcript, we were able to detect HAV sequence in the presence of a 200-million-fold excess of shellfish RNA. When intact virus was added to shellfish tissue before the isolation of RNA, the method was capable of detecting 10 viral RNA molecules in a reaction mixture.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen J. I., Ticehurst J. R., Purcell R. H., Buckler-White A., Baroudy B. M. Complete nucleotide sequence of wild-type hepatitis A virus: comparison with different strains of hepatitis A virus and other picornaviruses. J Virol. 1987 Jan;61(1):50–59. doi: 10.1128/jvi.61.1.50-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desenclos J. C., Klontz K. C., Wilder M. H., Nainan O. V., Margolis H. S., Gunn R. A. A multistate outbreak of hepatitis A caused by the consumption of raw oysters. Am J Public Health. 1991 Oct;81(10):1268–1272. doi: 10.2105/ajph.81.10.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami B. B., Glazer R. I. A simplified method for the production of recombinant baculovirus. Biotechniques. 1991 May;10(5):626–630. [PubMed] [Google Scholar]
- Jansen R. W., Siegl G., Lemon S. M. Molecular epidemiology of human hepatitis A virus defined by an antigen-capture polymerase chain reaction method. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2867–2871. doi: 10.1073/pnas.87.8.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Estes M. K., Metcalf T. G. Detection of hepatitis A virus by hybridization with single-stranded RNA probes. Appl Environ Microbiol. 1987 Oct;53(10):2487–2495. doi: 10.1128/aem.53.10.2487-2495.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Estes M. K., Metcalf T. G., Melnick J. L. Detection of hepatitis A virus in seeded estuarine samples by hybridization with cDNA probes. Appl Environ Microbiol. 1986 Oct;52(4):711–717. doi: 10.1128/aem.52.4.711-717.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N., Marchwicki R. H. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J Clin Microbiol. 1983 May;17(5):834–839. doi: 10.1128/jcm.17.5.834-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon S. M. Type A viral hepatitis. New developments in an old disease. N Engl J Med. 1985 Oct 24;313(17):1059–1067. doi: 10.1056/NEJM198510243131706. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Translation of messenger RNA in injected frog oocytes. Methods Enzymol. 1987;152:288–296. doi: 10.1016/0076-6879(87)52033-x. [DOI] [PubMed] [Google Scholar]
- Murphy L. D., Herzog C. E., Rudick J. B., Fojo A. T., Bates S. E. Use of the polymerase chain reaction in the quantitation of mdr-1 gene expression. Biochemistry. 1990 Nov 13;29(45):10351–10356. doi: 10.1021/bi00497a009. [DOI] [PubMed] [Google Scholar]
- Myers T. W., Gelfand D. H. Reverse transcription and DNA amplification by a Thermus thermophilus DNA polymerase. Biochemistry. 1991 Aug 6;30(31):7661–7666. doi: 10.1021/bi00245a001. [DOI] [PubMed] [Google Scholar]
- Nasser A. M., Metcalf T. G. Production of cytopathology in FRhK-4 cells by BS-C-1-passaged hepatitis A virus. Appl Environ Microbiol. 1987 Dec;53(12):2967–2971. doi: 10.1128/aem.53.12.2967-2971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B. H., Jansen R. W., Khanna B., Totsuka A., Nainan O. V., Siegl G., Widell A., Margolis H. S., Isomura S., Ito K. Genetic relatedness of hepatitis A virus strains recovered from different geographical regions. J Gen Virol. 1992 Jun;73(Pt 6):1365–1377. doi: 10.1099/0022-1317-73-6-1365. [DOI] [PubMed] [Google Scholar]
- Rotbart H. A. Enzymatic RNA amplification of the enteroviruses. J Clin Microbiol. 1990 Mar;28(3):438–442. doi: 10.1128/jcm.28.3.438-442.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler C. M., Fields H. A., Schable C. A., Meinke W. J., Maynard J. E. Adsorption, purification, and growth characteristics of hepatitis A virus strain HAS-15 propagated in fetal rhesus monkey kidney cells. J Clin Microbiol. 1986 Mar;23(3):434–440. doi: 10.1128/jcm.23.3.434-440.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. J., Estes M. K., Jiang X., Metcalf T. G. Concentration and detection of hepatitis A virus and rotavirus from shellfish by hybridization tests. Appl Environ Microbiol. 1991 Oct;57(10):2963–2968. doi: 10.1128/aem.57.10.2963-2968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]