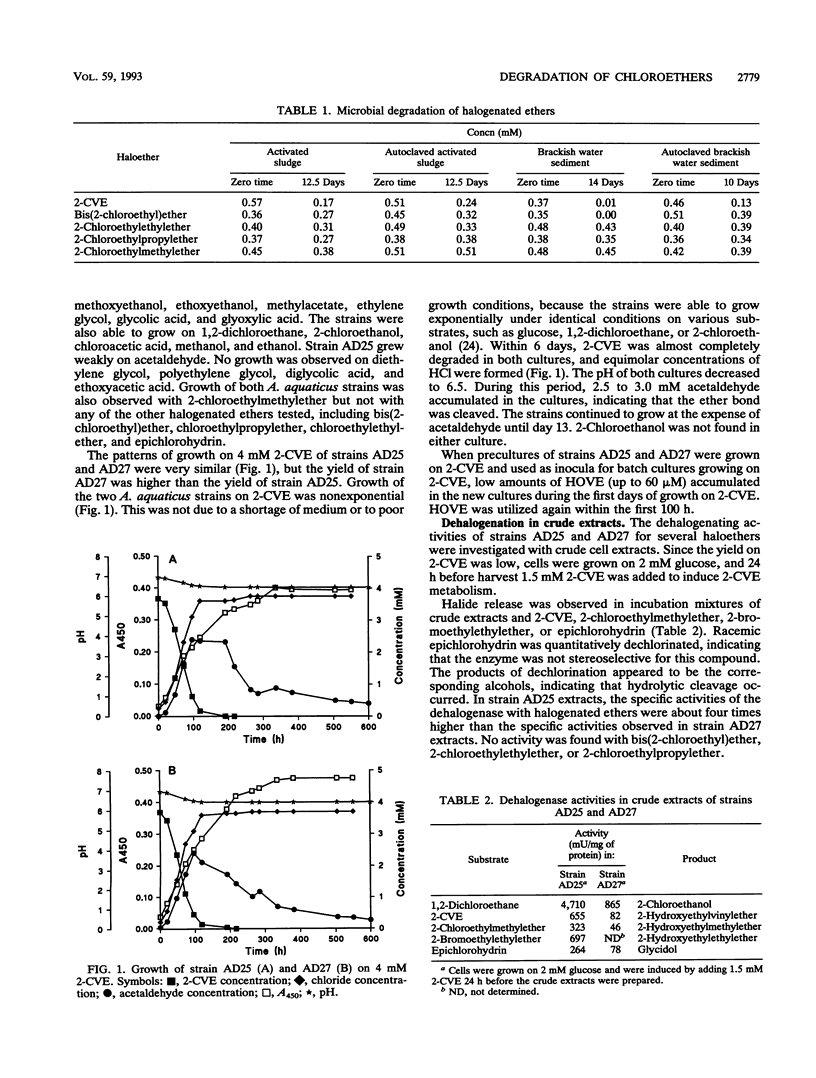
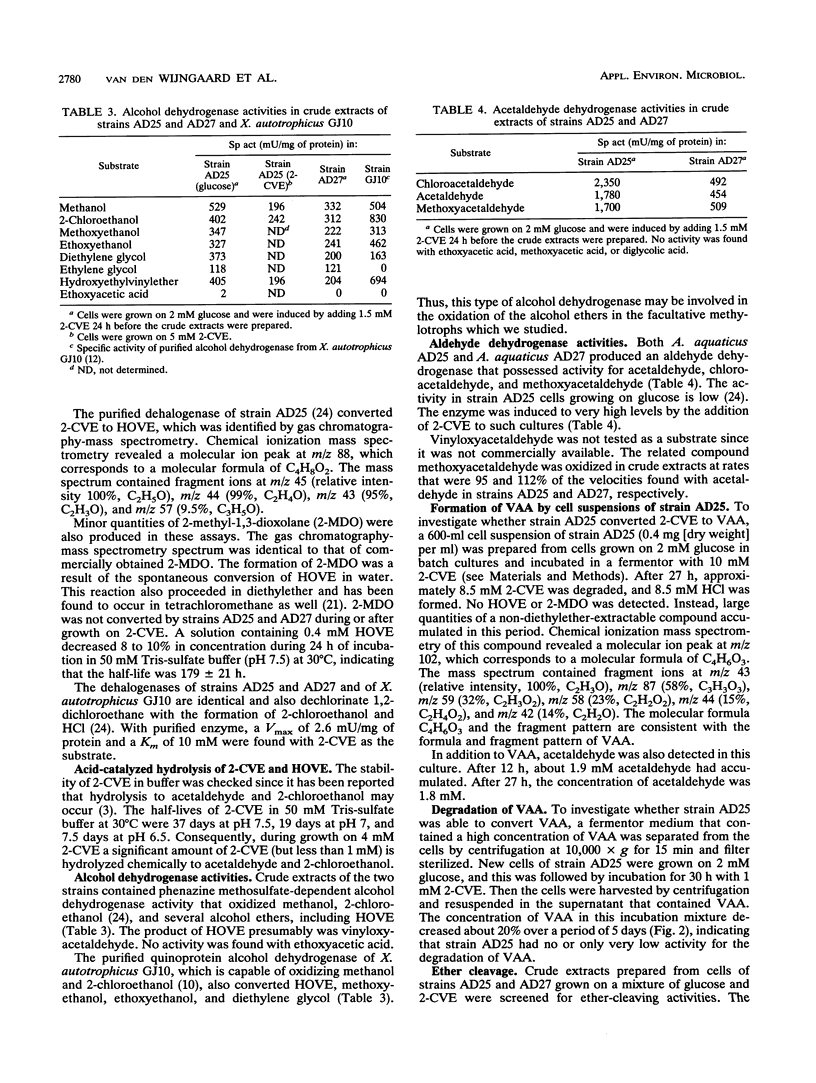
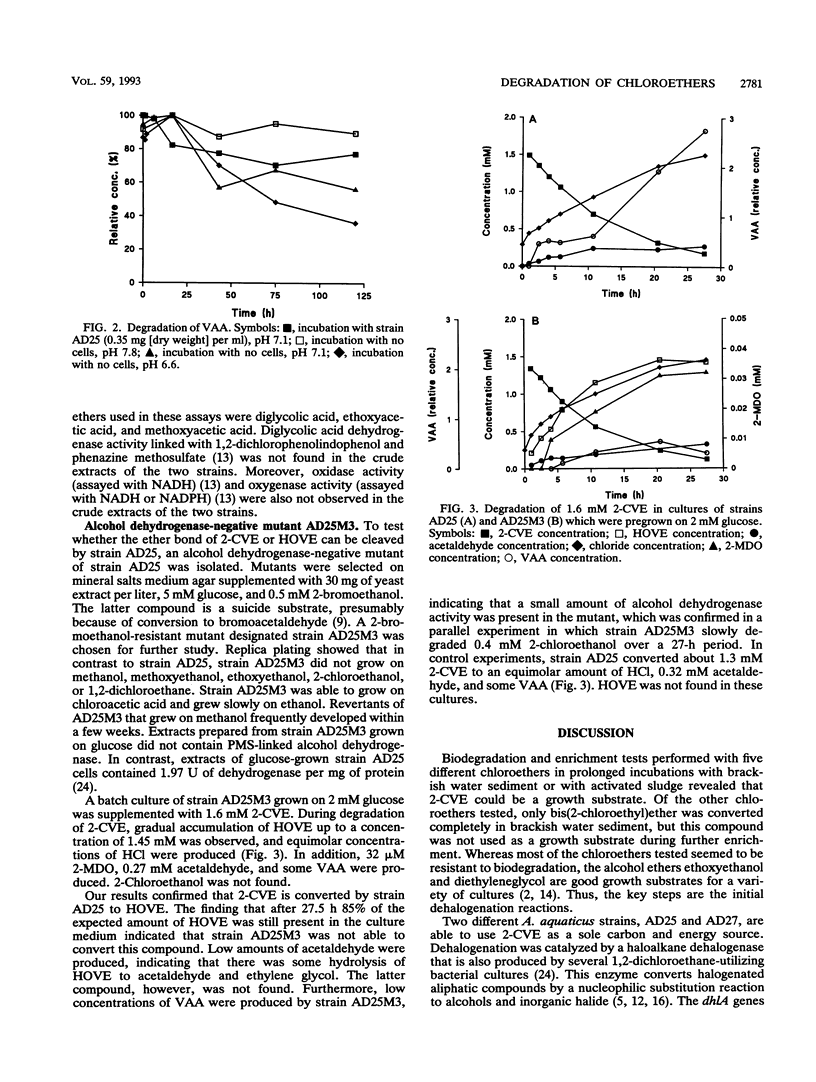
Abstract
Incubation of five different β-chloroethers with slurries prepared from brackish water sediment or activated sludge revealed that bis(2-chloroethyl)ether and 2-chloroethylvinylether (2-CVE) were biodegradable under aerobic conditions. After enrichment, two different cultures of Ancylobacter aquaticus that are capable of growth on 2-CVE were isolated. Both cultures were also able to grow on 1,2-dichloroethane. The cells contained a haloalkane dehalogenase that dehalogenated 2-CVE, 2-chloroethylmethylether, 2-bromoethylethylether, and epichlorohydrin. Experiments with cell extracts indicated that an alcohol dehydrogenase and an aldehyde dehydrogenase were also involved in the degradation of 2-CVE. This suggests that 2-CVE is metabolized via 2-hydroxyethylvinylether and vinyloxyacetaldehyde to vinyloxyacetic acid. Enzymatic ether cleavage was not detected. 2-CVE was also degraded by chemical ether cleavage, leading to the formation of 2-chloroethanol and acetaldehyde, both of which supported growth. We propose that A. aquaticus strains may be important for the detoxification and degradation of halogenated aliphatic compounds in the environment.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony C. Bacterial oxidation of methane and methanol. Adv Microb Physiol. 1986;27:113–210. doi: 10.1016/s0065-2911(08)60305-7. [DOI] [PubMed] [Google Scholar]
- Franken S. M., Rozeboom H. J., Kalk K. H., Dijkstra B. W. Crystal structure of haloalkane dehalogenase: an enzyme to detoxify halogenated alkanes. EMBO J. 1991 Jun;10(6):1297–1302. doi: 10.1002/j.1460-2075.1991.tb07647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines J. R., Alexander M. Microbial degradation of polyethylene glycols. Appl Microbiol. 1975 May;29(5):621–625. doi: 10.1128/am.29.5.621-625.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. H., Van den Wijngaard A. J., Pentenga M., Janssen D. B. Characterization of the epoxide hydrolase from an epichlorohydrin-degrading Pseudomonas sp. Eur J Biochem. 1991 Dec 18;202(3):1217–1222. doi: 10.1111/j.1432-1033.1991.tb16493.x. [DOI] [PubMed] [Google Scholar]
- Janssen D. B., Gerritse J., Brackman J., Kalk C., Jager D., Witholt B. Purification and characterization of a bacterial dehalogenase with activity toward halogenated alkanes, alcohols and ethers. Eur J Biochem. 1988 Jan 15;171(1-2):67–72. doi: 10.1111/j.1432-1033.1988.tb13759.x. [DOI] [PubMed] [Google Scholar]
- Janssen D. B., Jager D., Witholt B. Degradation of n-haloalkanes and alpha, omega-dihaloalkanes by wild-type and mutants of Acinetobacter sp. strain GJ70. Appl Environ Microbiol. 1987 Mar;53(3):561–566. doi: 10.1128/aem.53.3.561-566.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen D. B., Pries F., van der Ploeg J., Kazemier B., Terpstra P., Witholt B. Cloning of 1,2-dichloroethane degradation genes of Xanthobacter autotrophicus GJ10 and expression and sequencing of the dhlA gene. J Bacteriol. 1989 Dec;171(12):6791–6799. doi: 10.1128/jb.171.12.6791-6799.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen D. B., Scheper A., Dijkhuizen L., Witholt B. Degradation of halogenated aliphatic compounds by Xanthobacter autotrophicus GJ10. Appl Environ Microbiol. 1985 Mar;49(3):673–677. doi: 10.1128/aem.49.3.673-677.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai F., Yamanaka H. Biodegradation of polyethylene glycol by symbiotic mixed culture (obligate mutualism). Arch Microbiol. 1986 Nov;146(2):125–129. doi: 10.1007/BF00402338. [DOI] [PubMed] [Google Scholar]
- Keuning S., Janssen D. B., Witholt B. Purification and characterization of hydrolytic haloalkane dehalogenase from Xanthobacter autotrophicus GJ10. J Bacteriol. 1985 Aug;163(2):635–639. doi: 10.1128/jb.163.2.635-639.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradors N., Aguilar J. Efficient biodegradation of high-molecular-weight polyethylene glycols by pure cultures of Pseudomonas stutzeri. Appl Environ Microbiol. 1991 Aug;57(8):2383–2388. doi: 10.1128/aem.57.8.2383-2388.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wijngaard A. J., Reuvekamp P. T., Janssen D. B. Purification and characterization of haloalcohol dehalogenase from Arthrobacter sp. strain AD2. J Bacteriol. 1991 Jan;173(1):124–129. doi: 10.1128/jb.173.1.124-129.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wijngaard A. J., Wind R. D., Janssen D. B. Kinetics of bacterial growth on chlorinated aliphatic compounds. Appl Environ Microbiol. 1993 Jul;59(7):2041–2048. doi: 10.1128/aem.59.7.2041-2048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wijngaard A. J., van der Kamp K. W., van der Ploeg J., Pries F., Kazemier B., Janssen D. B. Degradation of 1,2-dichloroethane by Ancylobacter aquaticus and other facultative methylotrophs. Appl Environ Microbiol. 1992 Mar;58(3):976–983. doi: 10.1128/aem.58.3.976-983.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Waarde J. J., Kok R., Janssen D. B. Degradation of 2-chloroallylalcohol by a Pseudomonas sp. Appl Environ Microbiol. 1993 Feb;59(2):528–535. doi: 10.1128/aem.59.2.528-535.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]



