Abstract
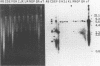
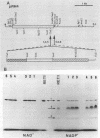
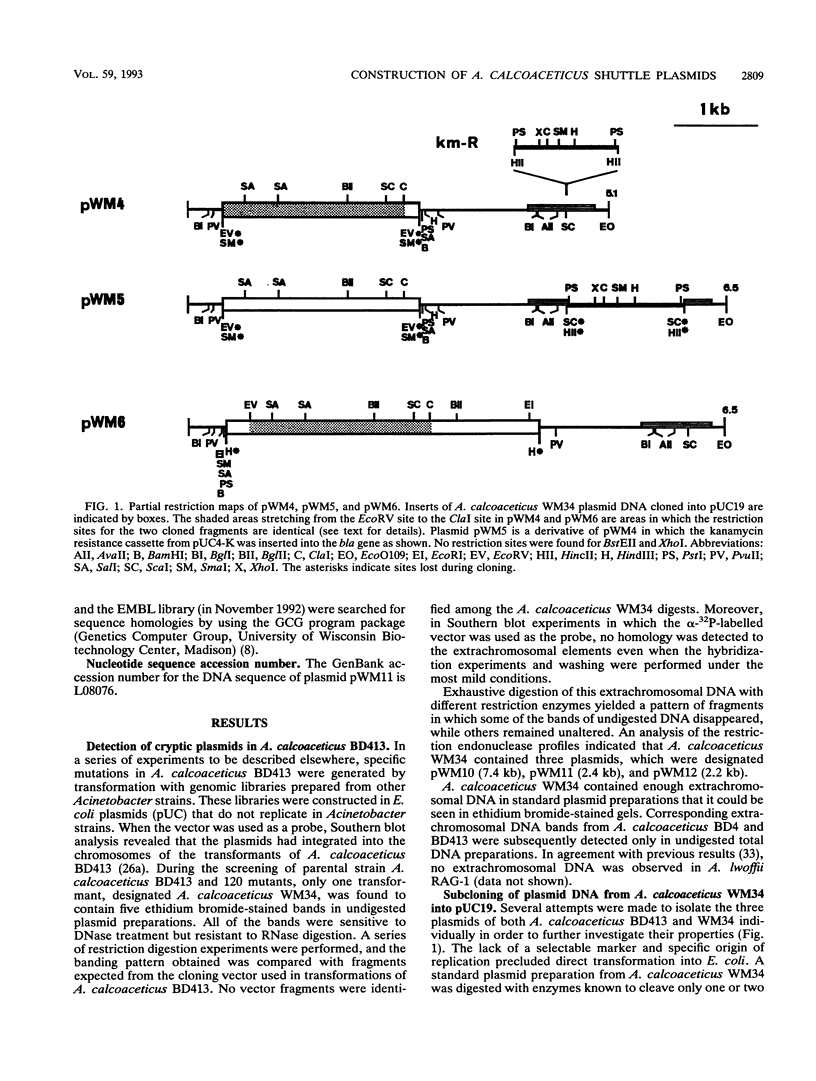
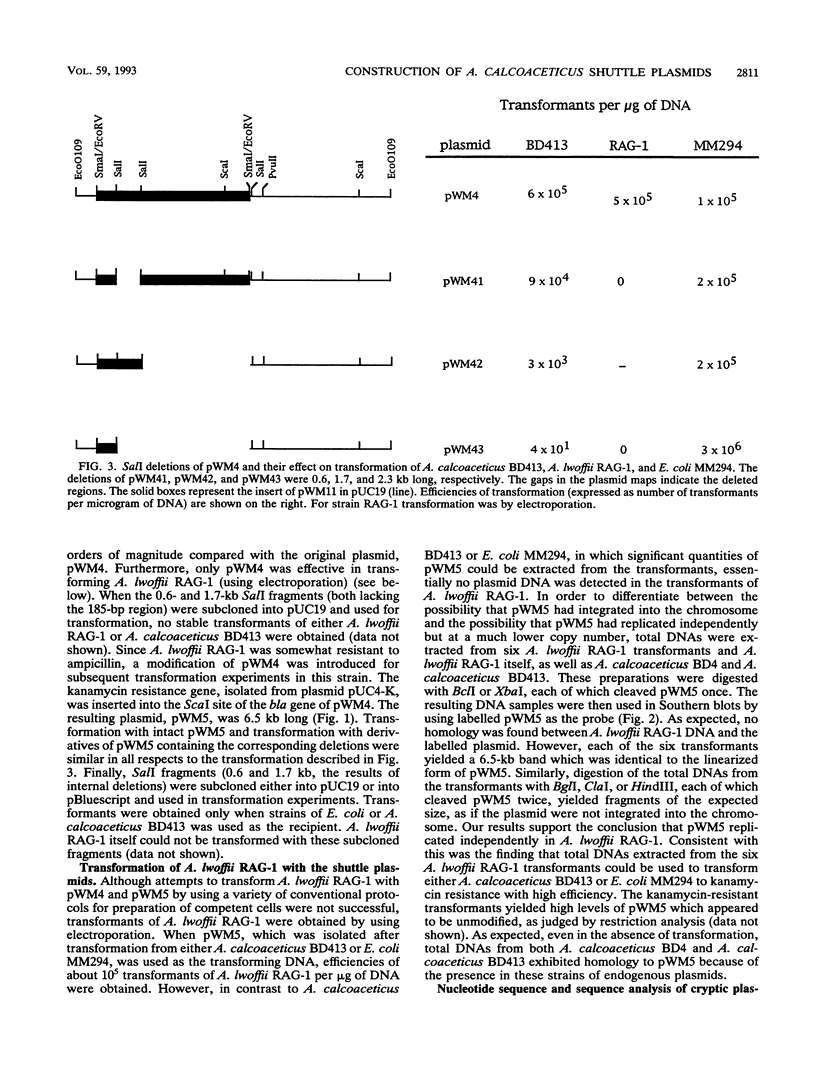
Three cryptic plasmids have been discovered in Acinetobacter calcoaceticus BD413. These three plasmids, designated pWM10 (7.4 kb), pWM11 (2.4 kb), and pWM12 (2.2 kb), exhibited extensive homology to one another, as shown by Southern blot hybridization and restriction site analysis data, and also hybridized with three plasmids having slightly different sizes detected in a second strain, A. calcoaceticus BD4. Plasmid pWM11 and a fragment of pWM10 were each subcloned into pUC19, yielding plasmids pWM4 and pWM6, respectively, and were used in a series of inter- and intraspecies transformation experiments. Both plasmids replicated as high-copy-number plasmids in A. calcoaceticus BD413, as well as in strains of Escherichia coli. However, when transformed into the oil-degrading strain Acinetobacter lwoffii RAG-1, both plasmids were maintained at low copy numbers. No modification of the plasmids was detected after repeated transfers between hosts. An analysis of a series of deletions demonstrated that (i) a 185-bp fragment of pWM11 was sufficient to permit replication of the shuttle plasmid in A. calcoaceticus BD413, (ii) the efficiency of transformation of A. calcoaceticus BD413 decreased according to the size of the deletion in the insert by up to 4 orders of magnitude, and (iii) the entire insert was required for transformation and replication in A. lwoffii RAG-1. The sequence of pWM11 contained several small (150- to 300-bp) open reading frames, none of which exhibited any homology to known DNA or protein sequences. In addition, a number of inverted and direct repeats, as well as six copies of the consensus sequence AAAAAAATA previously described for a cryptic plasmid from A. lwoffii (M. Hunger, R. Schmucker, V. Kishan, and W. Hillen, Gene 87:45-51, 1990), were detected. Cloning and expression of the alcohol dehydrogenase regulon from A. lwoffii RAG-1 were accomplished by using the Acinetobacter shuttle plasmid.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery L., Kaiser D. Construction of tandem genetic duplications with defined endpoints in Myxococcus xanthus. Mol Gen Genet. 1983;191(1):110–117. doi: 10.1007/BF00330897. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C. A rapid alkaline extraction method for the isolation of plasmid DNA. Methods Enzymol. 1983;100:243–255. doi: 10.1016/0076-6879(83)00059-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Cesareni G., Muesing M. A., Polisky B. Control of ColE1 DNA replication: the rop gene product negatively affects transcription from the replication primer promoter. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6313–6317. doi: 10.1073/pnas.79.20.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C. T., Niemela S. L., Miller R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2172–2175. doi: 10.1073/pnas.86.7.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couturier M., Bex F., Bergquist P. L., Maas W. K. Identification and classification of bacterial plasmids. Microbiol Rev. 1988 Sep;52(3):375–395. doi: 10.1128/mr.52.3.375-395.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie D. R., Kado C. I. Minimal region necessary for autonomous replication of pTAR. J Bacteriol. 1988 Jul;170(7):3170–3176. doi: 10.1128/jb.170.7.3170-3176.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hunger M., Schmucker R., Kishan V., Hillen W. Analysis and nucleotide sequence of an origin of DNA replication in Acinetobacter calcoaceticus and its use for Escherichia coli shuttle plasmids. Gene. 1990 Mar 1;87(1):45–51. doi: 10.1016/0378-1119(90)90494-c. [DOI] [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C., Miller J. R., Cress D. E., Wlodarczyk M., Manis J. J., Otten M. R. Nonintegrated plasmid-chromosome complexes in Escherichia coli. J Bacteriol. 1976 Aug;127(2):881–889. doi: 10.1128/jb.127.2.881-889.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. C., Miller J. R. Detection of nonintegrated plasmid deoxyribonucleic acid in the folded chromosome of Escherichia coli: physiochemical approach to studying the unit of segregation. J Bacteriol. 1975 Jan;121(1):165–172. doi: 10.1128/jb.121.1.165-172.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorowitz W., Clark D. Escherichia coli mutants with a temperature-sensitive alcohol dehydrogenase. J Bacteriol. 1982 Nov;152(2):935–938. doi: 10.1128/jb.152.2.935-938.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahairas G. G., Minion F. C. Transformation of Mycoplasma pulmonis: demonstration of homologous recombination, introduction of cloned genes, and preliminary description of an integrating shuttle system. J Bacteriol. 1989 Apr;171(4):1775–1780. doi: 10.1128/jb.171.4.1775-1780.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukata H., Tomizawa J. Effects of point mutations on formation and structure of the RNA primer for ColE1 DNA replication. Cell. 1984 Feb;36(2):513–522. doi: 10.1016/0092-8674(84)90244-7. [DOI] [PubMed] [Google Scholar]
- Moser D. R., Campbell J. L. Characterization and complementation of pMB1 copy number mutant: effect of RNA I gene dosage on plasmid copy number and incompatibility. J Bacteriol. 1983 May;154(2):809–818. doi: 10.1128/jb.154.2.809-818.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Plasmid incompatibility. Microbiol Rev. 1987 Dec;51(4):381–395. doi: 10.1128/mr.51.4.381-395.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P. G., Allon R., Mevarech M., Mendelovitz S., Sato Y., Gutnick D. L. Cloning and expression in Escherichia coli of an esterase-coding gene from the oil-degrading bacterium Acinetobacter calcoaceticus RAG-1. Gene. 1989 Mar 15;76(1):145–152. doi: 10.1016/0378-1119(89)90016-4. [DOI] [PubMed] [Google Scholar]
- Reisfeld A., Rosenberg E., Gutnick D. Microbial degradation of crude oil: factors affecting the dispersion in sea water by mixed and pure cultures. Appl Microbiol. 1972 Sep;24(3):363–368. doi: 10.1128/am.24.3.363-368.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusansky S., Avigad R., Michaeli S., Gutnick D. L. Involvement of a plasmid in growth on and dispersion of crude oil by Acinetobacter calcoaceticus RA57. Appl Environ Microbiol. 1987 Aug;53(8):1918–1923. doi: 10.1128/aem.53.8.1918-1923.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. T., Finnerty W. R. Insertional specificity of transposon Tn5 in Acinetobacter sp. J Bacteriol. 1984 Feb;157(2):607–611. doi: 10.1128/jb.157.2.607-611.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer J. T., van Tuijl J. J., Finnerty W. R. Transformation and mobilization of cloning vectors in Acinetobacter spp. J Bacteriol. 1986 Jan;165(1):301–303. doi: 10.1128/jb.165.1.301-303.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M. E., Finnerty W. R. Alcohol dehydrogenases in Acinetobacter sp. strain HO1-N: role in hexadecane and hexadecanol metabolism. J Bacteriol. 1985 Dec;164(3):1017–1024. doi: 10.1128/jb.164.3.1017-1024.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H., JUNI E. Pathways for biosynthesis of a bacterial capsular polysaccharide. I. Carbohydrate metabolism and terminal oxidation mechanisms of a capsuleproducing coccus. J Bacteriol. 1961 May;81:694–703. doi: 10.1128/jb.81.5.694-703.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR W. H., JUNI E. Pathways for biosynthesis of a bacterial capsular polysaccharide. I. Characterization of the organism and polysaccharide. J Bacteriol. 1961 May;81:688–693. doi: 10.1128/jb.81.5.688-693.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]