Abstract
We propose a rational approach to the generation of live viral vaccines: alteration of virally encoded type I IFN antagonists to attenuate virulence while retaining immunogenicity. We have explored this concept by using the influenza virus. Previously we have shown that the NS1 protein of influenza A virus possesses anti-IFN activity. We now present evidence that influenza A and B viruses encoding altered viral NS1 proteins are highly attenuated in the mouse host, yet provide protection from challenge with wild-type viruses.
Influenza virus is a formidable pathogen, killing thousands of people per year in the United States alone. In addition, widespread morbidity caused by the virus has enormous social and economic impact. Therefore, ongoing vaccine intervention is imperative. By anticipating which influenza A and B virus strains will circulate during a given year and then designing a vaccine to induce protection against these strains, we are able to take a rational approach to defending ourselves against this virus.
Inactivated (killed) influenza virus preparations are the only influenza vaccines currently licensed in the United States. These multisubunit vaccines are designed to elicit humoral immunity directed against current influenza A and B virus strains. Another promising approach to vaccination is the use of cold-adapted live attenuated influenza viruses (reviewed in ref. 1). Obtained by multiple passage at low temperature, these cold-adapted viruses are growth restricted to the upper respiratory tract. Cold-adapted viruses have been reported to induce not only humoral responses against homotypic influenza virus but also crossreactive cell-mediated cytotoxicity (2, 3). Additional advantages of a live viral vaccine include the ease of intranasal administration, induction of mucosal immunity, and cost effectiveness.
We propose an alternative rational approach to the design of live virus vaccines by alteration of viral IFN antagonists. We have demonstrated previously that the influenza A virus NS1 protein exhibits IFN antagonist activity, allowing influenza virus to replicate in IFN competent systems (4). For the present study, we determined the vaccine potential of several influenza A and B viruses encoding altered NS1 proteins. These viruses show significant growth attenuation in immunologically mature embryonated chicken eggs and in BALB/c mice. Furthermore, we demonstrate that immunization of mice with NS1-altered influenza viruses provides protective immunity in mice against the replication and/or pathogenicity of wild-type influenza virus. The model presented here may be applicable to the rational generation of vaccines for influenza and other viruses with defined IFN antagonists.
Materials and Methods
Viruses.
NS1–99 virus was generated by ribonucleoprotein transfection (5) by using the 25A-1 helper influenza virus as described for delNS1 virus (4). NS1–99 virus contains a 154-nt insertion between nucleotide positions 324–478 of the NS1–99 viral NS gene. This insertion creates a frame shift and results in the generation of NS1 protein containing the first 99 amino acids of wild-type NS1, with three additional C-terminal amino acids (HisAspSer). This insertion arose as a PCR artifact during repair of a pUC19-T3/NS construct by site-directed mutagenesis and contains seven 22-nt repeats (5′-CATGACTCTTGAGGAAATGTCA-3′). Influenza B/Yamagata/1/73 clone 201 (B/201) and influenza B/Yamagata AWBY-234 (B/234) have been described previously (6–9).
Influenza A viruses were grown in embryonated chicken eggs between the ages of 6 and 14 days old (SPAFAS, Preston, CT) at 37°C for 48 h. Influenza B viruses were grown in embryonated chicken eggs at 35°C for 72 h. One hundred plaque-forming units (pfu) of virus were injected into the allantoic cavity of each egg. Allantoic fluid from influenza A or B virus-infected eggs was serially diluted in PBS and assayed for hemagglutination (HA) of chicken red blood cells (0.5%; Truslow Farms, Chestertown, MD) in 96-well plates. Plaque assay of influenza A or B virus stocks was performed on Madin–Darby canine kidney cells (MDCK) cells in the presence of 2 μg/ml trypsin (Difco) at 37°C (influenza A viruses) or 35°C (influenza B viruses). It should be noted that the MDCK plaque size of delNS1 virus is markedly reduced as compared with wild-type PR8 virus. This is likely to be a reflection of major difference in the permissiveness of MDCK cells for the replication of these two viruses (4).
Immunoprecipitation of NS1–99 from Infected Cells.
Dishes (3.5 cm) of 70% confluent MDCK cells were infected [multiplicity of infection (MOI) = 10] with wild-type PR8 virus, the recombinant NS1–99 virus, or mock infected with PBS for 1 h at room temperature. Cells were then incubated in 1 ml labeling media (MEM–cys/met + 250 μCi 35S-cys/met) for 8 h at 37°C and lysed on ice in 200 μl RIPA buffer. Lysates were clarified by centrifugation for 10 min at 12,000 × g. Cell supernatant was precipitated for 2 h at 4°C by using a polyclonal antibody (2 μl) against the influenza A viral NS1 protein (8). Fifty microliters of Protein A Sepharose beads was added to mixture and antibody complexes were pelleted after incubation at 4°C for 1 h. Pellets were washed twice with RIPA buffer (0.15 mM NaCl/0.05 mM Tris⋅HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS), and half of the samples were loaded onto 17.5% SDS/PAGE gel. Separated bands were visualized by autoradiography.
Western Blot Analysis.
Thirty-five-millimeter dishes of confluent Vero cells were mock infected or infected at an MOI of 2 with either NS1–99, delNS1, or PR8 virus for the indicated time point. Cell extracts were made in RIPA buffer, and 10% were loaded on an SDS–polyacrylamide gel. Separated proteins were probed for Western analysis with a rabbit polyclonal against the viral nucleoprotein or a rabbit polyclonal against the nuclear export protein (NEP) (10).
Immunization and Challenge of Mice.
BALB/c mice are an established model for influenza viral infection (11–14). Four-week-old female BALB/c mice (Taconic Farms) were sedated with ether and infected intranasally with indicated amounts of influenza A or B virus (50 μl volume). Recovery from ether occurred within 1–2 min after infection. For viral lung titers, mice were killed at either day 3 or day 6. Lungs were homogenized and resuspended in sterile PBS (100 mg lung tissue per 1 ml PBS) and titered on MDCK cells in the presence of 2 μg/ml trypsin.
Four weeks after immunization, mice were challenged by intranasal infection with either wild-type PR8 virus (105 or 5 × 106 pfu) or wild-type B/Yamagata virus (3 × 105 pfu), as indicated in Tables 1 and 2.
Table 1.
Survival of mice immunized with NS1 attenuated influenza A viruses and challenged with wild-type influenza A/PR8 virus
| Group | Survivors
|
||||
|---|---|---|---|---|---|
| Immunization
|
After immunization | After challenge*
|
|||
| Virus | Dose, pfu | 1 × 105pfu | 5 × 106pfu | ||
| A | A/delNS1 | 1.0 × 106 | 9/9 | 5/5 | 4/4 |
| B | 3.3 × 104 | 5/5 | 0/5 | ND | |
| C | A/NS1-99 | 1.0 × 106 | 8/10 | 5/5 | 3/3 |
| D | 3.3 × 104 | 9/10 | 3/5 | 4/4 | |
| E | A/PR8 | 2.0 × 103 | 1/5 | ND | 1/1 |
| F | PBS | 0 | 6/6 | 0/6 | ND |
Mice were challenged with 1 × 105 pfu (100 LD50) or with 5 × 106 pfu (5,000 LD50) influenza A/PR8 virus 4 wk after immunization. ND, not determined.
Table 2.
Lung titers of mice immunized with NS1 attenuated influenza B viruses and challenged with wild-type influenza B/Yamagata virus
| Virus | Dose, pfu | Lung titers before challenge, pfu/ml
|
Lung titers after challenge* | |
|---|---|---|---|---|
| Day 3 | Day 6 | |||
| B/234 | 3 × 105 | 40, <10, <10 | <10, <10, <10 | <10, <10, <10, <10, <10, <10 |
| B/201 | 3 × 105 | 60, 30, <10 | <10, <10, <10 | <10, <10, <10, <10, <10, <10 |
| B/Yam | 3 × 105 | 3.0 × 104, 2.0 × 104, 1.0 × 104 | <10, <10, <10 | <10, <10, <10, <10, <10, <10 |
| PBS | 0 | ND | ND | 2.5 × 104, 1.7 × 104 |
Mice were challenged with 3 × 105 pfu B/Yamagata virus 4 wk after immunization. Lung titers were taken 3 days after challenge.
All procedures were in accordance with National Institutes of Health guidelines for the care and use of laboratory animals.
ELISA.
To quantitate the amounts of virus-specific antibodies present in immunized mice, ELISA analysis of the reactivity of diluted (1:1,000) serum against purified viral antigen was performed. Blood was drawn from mice 4 wk after immunization and before challenge with wild-type virus. Blood was incubated at 4°C for 2 h and spun at 200 × g for 10 min. Supernatant (serum) was removed and stored at −20°C. Fifty microliters of sucrose gradient purified influenza A/PR8 or B/Yamagata virus (25 mg/ml) was used to coat 96-well ELISA dishes (Immulon4, Dynex, Chantilly, VA). After PBS wash, coated wells were blocked with PBS 1% BSA and incubated with diluted (1:1,000) serum. After 1 h incubation with serum at room temperature, wells were rinsed with PBS and incubated with a secondary anti-mouse IgG peroxidase (Boehringer-Mannheim). Rinsed wells were incubated with colorimetric substrate (2,2′-azino-di-[3-ethylbenzthiazoline sulfonate], Boehringer-Mannheim) for 20′ and read with ELISA reader (OD595, Bio-Tek Instruments, Burlington, VT).
Enzyme-Linked Immunospot (ELISPOT) Assay.
The ELISPOT assay used to detect antigen-specific cytotoxic T lymphocytes was performed as described previously (15, 16). This assay measures the number of IFN-γ-producing spleen cells in cells exposed to MHC class I-presented NP peptide. Ninety-six-well nitrocellulose plates (Milititer HA, Millipore) were coated with 7 μg/ml of a monoclonal antibody against mouse IFN-γ (no. R4–6A2, PharMingen) in 75 μl PBS and incubated at room temperature overnight. Wells were washed six times with culture medium and incubated with DMEM supplemented with 10% FCS for 1 h at 37 °C. Pooled splenocytes from immunized mice were added to antibody-coated wells in serial dilutions. P815 cells (a mastocytoma cell line that expresses only MHC class I molecules) were used as antigen-presenting cells. P815 cells (1 × 105 cells/ml) were pulsed with 1 × 10−6 M of the synthetic NP peptide TYQRTRALV for 1 h at 37°C. After repeated washings with culture medium, cells were treated with 50 μg/ml mitomycin C (Sigma) for 1 h. NP-peptide treated P815 cells were added to each well. As a control, untreated P815 cells were used. Plates were incubated for 24 h at 37°C with 5% CO2 and then washed extensively with PBS + 0.05% Tween-20 (PBS/T). Wells were incubated with a solution of 2 μg/ml biotinylated anti-mouse IFN-γ monoclonal antibody (XGM1.2, PharMingen) in PBS/T for 1 h at room temperature. Plates were washed with PBS/T and incubated with peroxidase-labeled streptavidin (1:1,000; Kirkegaard & Perry Laboratories) for 1 h at room temperature. Wells were washed with PBS/T and PBS, and 1 μg/ml substrate (3,3′-diaminobenzidine tetrahydrochoride dihydrate in 50 mM Tris⋅HCl, pH 7.5, containing 0.015% hydrogen peroxide) was added. Spots in each well were counted with the aid of a microscope.
Results
Growth of NS1-Altered Influenza A Viruses in Embryonated Eggs.
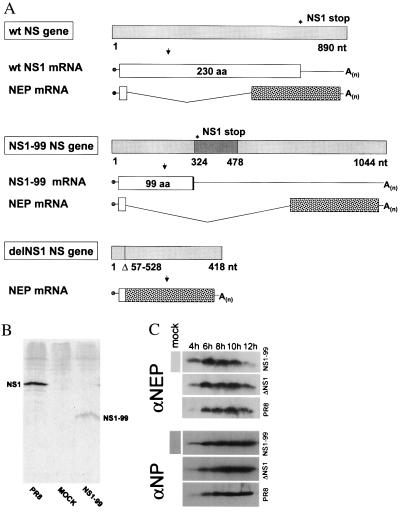
DelNS1 and NS1–99 viruses were generated by ribonucleoprotein transfection of altered viral influenza NS genes by using the temperature-sensitive helper virus 25A-1 as described previously (Fig. 1A) (4). DelNS1 virus lacks the NS1 gene, and the NS1–99 virus encodes a mutant NS1 protein containing only the N-terminal 99 amino acids of wild-type NS1, creating an NS1 protein of approximately 11.2 kDa (Figure 1B). By contrast, the wild-type virus encodes an NS1 protein of 230 amino acids. We infected Vero cells, a cell line that is known to lack the type I IFN loci (17, 18), with NS1–99, delNS1, or PR8 virus at an MOI of 2. Despite the alterations in the NS gene, all three of these influenza A viruses express comparable levels of the viral NEP (NS2), the second protein encoded by the viral NS gene (Fig. 1 A and C).
Figure 1.
Wild-type A/PR8 influenza virus and derivative viruses containing alterations in the viral NS1 ORFs. (A) Schematic representation of the NS genes and gene transcripts for wild-type influenza A PR8 virus and transfectant NS1–99 and delNS1 viruses. Viral NS genes are indicated by light-gray boxes, with nucleotide length indicated in numbers below the gene segments. Stop codons for PR8 and NS1–99 NS1 ORFs are indicated by an asterisk above the viral genes. A 154-nt insertion in the NS1–99 gene is shown as a dark-gray box, and the 471-nt deletion of the delNS1 NS gene segment is represented by a vertical line with the deleted nucleotides listed below the viral gene. Viral NS1 ORFs are represented by white boxes with the amino acid length indicated within each box. Viral NEP mRNAs are also shown, with white boxes indicating the in-frame mRNA sequence shared between viral NS1 and NEP ORFs, and spotted boxes representing the unique ORFs of the viral NEP mRNA transcripts. Influenza A/PR8 and A/delNS1 viral NS genes have been described previously (4, 35) and are shown together with the A/NS1–99 NS gene for comparison. (B) Immunoprecipitation of NS1 protein from NS1–99 infected cells. MDCK cells were infected with PR8 or NS1–99 virus as described in Materials and Methods. 35S-met-cys-labeled NS1 protein was immunoprecipitated from infected cell extracts by using rabbit polyclonal antisera against the NS1 protein and separated on a 17.5% SDS gel. Proteins were visualized by autoradiography. (C) Western blot analysis of NEP expression. Vero cells were infected for the indicated time points at an MOI of 2 with either NS1–99, delNS1, or PR8 virus. Cell extracts were probed with an antibody against the viral NEP (Upper) or NP (Lower) as described in Materials and Methods.
Others have demonstrated that the IFN inducibility of both cultured chicken embryo cells and the intact embryonated egg increases with age (19–21). Specifically, Morahan and Grossberg established a strong correlation between age-related IFN induction and resistance of older intact chicken embryos to influenza (NWS) virus infection (21). We wished to determine the growth properties of influenza A and B viruses encoding either wild-type or altered NS1 proteins in eggs of varying age to examine the relationship between alteration in the NS1 protein and its functionality as an IFN antagonist. Our prediction was that alteration of this virally encoded IFN antagonist would compromise the ability of a virus to grow in older eggs but would still allow growth in younger eggs.
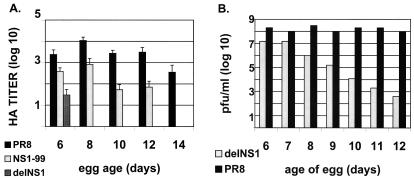
Equivalent doses of influenza A/PR8, NS1–99, or delNS1 viruses were injected into the allantoic cavity of embryonated chicken eggs ranging in age from 6 to 14 days old. Infected eggs were incubated at 37°C for 48 h. Allantoic fluid was harvested and assayed for HA activity as described in Materials and Methods. As shown in Fig. 2A, wild-type influenza A/PR8 virus was able to grow to high HA titers in embryonated eggs regardless of age, whereas growth of an isogenic virus lacking the viral NS1 gene (delNS1 virus) is compromised in its ability to grow to high HA titers in eggs older than 6 days. The NS1–99 virus showed intermediate growth characteristics, growing to high HA titers in 6- and 8-day-old eggs, and to moderate HA titers in 10- and 12-day-old eggs. The NS1–99 virus was unable to grow in 14-day-old eggs and consistently grew to titers that were slightly lower than the wild-type parent virus (Figure 2A). Virus titers from allantoic fluid of eggs infected with delNS1 or PR8 virus were also measured by plaque assay (Fig. 2B). Yield of delNS1 virus decreased with the age of the infected egg, corresponding to the reduction observed in HA titers, with 6- and 7-day-old eggs yielding a maximum of 1 × 107 pfu/ml allantoic fluid. PR8 virus consistently grew to titers greater than 1 × 108 pfu/ml, regardless of the egg age.
Figure 2.
Growth characteristics of wild-type PR8, NS1–99, and delNS1 viruses in embryonated eggs. (A) One hundred pfu of virus was injected into the allantoic cavity of embryonated eggs of varying age and incubated for 48 h at 37°C. Allantoic fluid was harvested and titrated by HA assay as described in Materials and Methods. HA titers were not detected for delNS1 virus at days 8, 10, 12, or 14. NS1–99 infection did not give detectable HA titers at day 14. Graph represents the average reciprocal HA dilution of two to six eggs for each virus. (B) Embryonated eggs were infected with either PR8 or delNS1 virus as in A, and allantoic fluid was titrated on MDCK cells as described in Materials and Methods.
Vaccination of Mice with NS1–99 and delNS1 Viruses.
Influenza A/delNS1 virus has been shown previously to be nonpathogenic in wild-type mice. When mice were inoculated intranasally with 5 × 104 pfu of delNS1 virus, all survived without showing any weight loss (4). We extended our pathogenicity studies by using the NS1–99 virus and examined whether delNS1 and/or NS1–99 viruses induced protective immune responses. As summarized in Table 1, 4-wk-old female BALB/c mice were inoculated with various dilutions of either delNS1 (groups A-B) or NS1–99 (groups C-D) virus and monitored for 4 wk. Mice infected with 2 × 103 pfu (2 LD50) of wild-type influenza PR8 virus or mock infected with PBS were included as controls. All mice inoculated with delNS1 virus survived, even at the highest inoculum (1 × 106 pfu). Eight of ten mice inoculated with the highest dose of NS1–99 virus (group C) survived, and mice immunized with a lower dose (3.3 × 104 pfu, group D) demonstrated a 90% survival rate. In contrast, only one of five mice survived after administration of 2 × 103 pfu of wild-type PR8 (group E). To determine virus replication levels in lungs of infected mice, three groups of six mice each were also infected with 1 × 104 pfu of either PR8, NS1–99, or delNS1 virus and killed at day 3 or day 6. Neither NS1–99 nor delNS1 virus-infected mice showed detectable lung titers at either day 3 or day 6 (<10 pfu/ml), whereas wild-type PR8 virus-infected mice had average lung titers of 2 × 106 pfu/ml and 3 × 104 pfu/ml at day 3 and day 6, respectively. One of six mice inoculated with 1 × 104 pfu PR8 virus died before lung titration at day 6.
Four weeks after immunization, surviving mice were challenged with 1 × 105 pfu (100 LD50) or 5 × 106 pfu (5,000 LD50) of wild-type PR8 virus. As shown in Table 1, mice immunized with 1 × 106 pfu of either delNS1 (group A) or NS1–99 (group C) were completely protected against lethal challenge with 100 or even 5,000 LD50 of influenza A/PR8 virus. Reduction in the immunizing dose of NS1–99 virus to 3.3 × 104 pfu (group D) resulted in 78% protection, with 7 of 9 mice surviving 2 wk after challenge. Reduction in the immunizing dose of delNS1 virus to 3.3 × 104 pfu (group B) resulted in a loss of protection against lethal (100 or 5,000 LD50) PR8 virus challenge. None of the mice mock immunized was protected against wild-type challenge, and the one mouse surviving PR8 virus infection was protected when reexposed to this virus.
Immune Responses in Mice Vaccinated with NS1–99 and delNS1 Viruses.
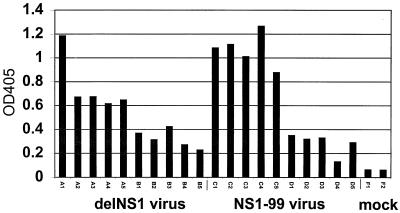
Before challenge with wild-type influenza A/PR8 virus, serum samples were taken from delNS1 and NS1–99 immunized mice (five mice each from groups A–D in Table 1) and from mock-immunized mice (group F) and analyzed for reactivity against purified influenza A/PR8 virus by ELISA. As shown in Fig. 3, immunization with 1 × 106 pfu of either delNS1 virus (group A; mice A1-A5) or NS1–99 virus (group C; mice C1-C5) gave measurable antibody titers against wild-type virus. Reduction in the immunizing dose of either delNS1 virus (groups B; mice B1-B5) or NS1–99 virus (groups D; mice D1-D5) resulted in a concomitant reduction in PR8-reactive immune sera. As expected, serum from mice inoculated with PBS (group F) did not react with purified influenza A/PR8 virus.
Figure 3.
Influenza A-specific antibody titers of sera taken from mice immunized with delNS1 virus or NS1–99 virus. Serum samples were tested from sample groups A–D or from mock-immunized mice (group F) as indicated in Table 1. Samples were taken from mice 4 wk after immunization with primary virus and before challenge with wild-type PR8 virus. Samples were tested for their reactivity to sucrose purified influenza A/PR8. OD405 readings for serum diluted 1:1,000 are shown.
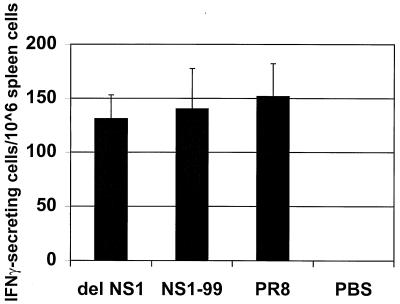
We also examined the production of NP peptide-specific T cells in delNS1 or NS1–99 virus immunized mice. Groups of three BALB/c mice were immunized intranasally with 1 × 103 pfu of either delNS1, NS1–99, or PR8 virus. At day 10, spleen cells were harvested and analyzed by enzyme-linked immunospot for reactivity to an influenza virus NP peptide as previously described (15, 16). As shown in Fig. 4, mice immunized with either delNS1 or NS1–99 virus stimulated the production of NP-peptide-specific spleen cells to levels comparable to those seen in mice exposed to wild-type influenza A/PR8 virus. As expected, intranasal inoculation of mice with PBS did not induce the production of spleen cells reactive to influenza A NP peptide. These results are indicative of at least abortive viral replication, as immunization with inactivated virus has been shown not to induce a detectable cytotoxic T lymphocyte response (22).
Figure 4.
NP-peptide specific spleen cells from mice immunized with delNS1 virus or NS1–99 virus. Spleen cells from mice immunized with 1 × 103 pfu of the indicated virus were harvested 10 days after immunization and assayed for influenza NP peptide-specific spleen cells, as described in Materials and Methods. Spots corresponding to IFN-γ-secreting cytotoxic T lymphocytes were counted at two different cell dilutions and averaged for each virus group. Results indicate the average number of spots of two different spleen cell dilutions, extrapolated to 1 × 106 cells. Spleen cells were harvested from three mice for each group, with the exception of PR8-immunized mice. One of three mice infected with 1 × 103 pfu of PR8 virus survived immunization and was killed for spleen cell analysis at day 10.
Growth of NS1-Attenuated Influenza B Viruses in Embryonated Eggs.
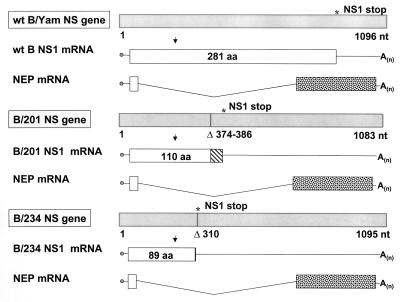
Influenza B/201 and influenza B/AWBY/234 (B/234) viruses are laboratory variants of influenza B/Yamagata/1/73 that contain alterations in the NS1 ORF as compared with the NS1 ORF of wild-type influenza B/Yamagata (6, 7). As shown in Fig. 5, nucleotide deletions in the NS gene of either B/201 or B/234 create frame shifts that result in the generation of shortened NS1 proteins. The influenza B/201 and B/234 viral NS1 proteins contain, respectively, 110 and 89 amino acids corresponding to the N terminus of the B/Yamagata viral NS1 protein.
Figure 5.
Wild-type B/Yamagata/1/73 influenza virus and derivative viruses containing alterations in the viral NS1 ORFs. Influenza B/Yamagata NS genes and mRNA transcripts are schematically represented as in Fig. 1. Viral NS gene segments are represented by light-gray boxes and viral NS1 ORFs by white boxes. A 13-nt deletion in the B/201 NS gene is indicated by a vertical bar. Seventeen amino acids resulting from a frameshift in the B/201 NS1 ORF are indicated by a hatched bar (8). Deletion of nucleotide number 310 in the B/234 NS gene segment results in a frameshift encoding one leucine and then a stop codon, represented by a vertical bar. The B/234 viral NS1 protein also contains a glu → val change at amino acid position 66 (not shown). B/Yamagata, B/201, and B/234 NS genes have been described previously, (6, 8) and are shown here together for comparison of the NS1 ORFs. Length of B/AWBY NS gene as predicted from the literature is indicated (6).
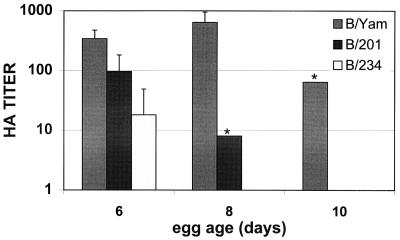
We hypothesized that the NS1 of influenza B virus would show IFN antagonist activity in vivo. We therefore examined whether influenza B/Yamagata virus derivatives that code for shortened NS1 proteins would show growth attenuation in older immunologically mature embryonated eggs as compared with wild-type influenza B/Yamagata virus. Both B/201 and B/234 viruses grow in 6-day-old eggs albeit to lower titers than B/Yamagata virus (Fig. 6). However, whereas B/Yamagata grew equally well in 8-day-old eggs, growth of the other two viruses was markedly reduced in embryos of this age.
Figure 6.
Growth characteristics of wild-type B/Yamagata, B/201, and B/234 viruses in embryonated eggs. One hundred pfu of influenza B viruses was injected into the allantoic cavity of embryonated eggs and incubated at 35°C for 72 h. Allantoic fluid was then harvested and subjected to HA analysis. Graph represents the average reciprocal HA dilution of two to six eggs for each virus. Asterisks indicate that two eggs were tested and gave the same HA titer. Influenza B/234 virus showed no HA titers in 8- and 10-day-old eggs.
Vaccination of Mice with B/201 and B/234 Viruses.
We also compared the virulence of influenza B viruses in BALB/c mice. Because influenza B/Yamagata virus is not mouse adapted and does not cause disease or death in mice, we examined viral lung titers as an index of the ability of different viruses to replicate within the mouse host. We found that at 3 days after infection, B/201 and B/234 viruses showed more than two logs reduction in viral titers as compared with the wild-type B/Yamagata virus (Table 2). By 6 days after infection, mice in all infected groups had cleared the virus, and lung titers were below the limit of detection by plaque assay. In a separate experiment, we tested whether previous immunization of mice was able to protect against infection by wild-type B/Yamagata virus. Mice immunized with either B/201 or B/234 viruses were indeed protected, as shown by lack of replication at day 3 by the wild-type virus in the lungs of these mice.
Antibody Responses in Mice Vaccinated with B/201 and B/234 Viruses.
Four weeks after immunization and before challenge with wild-type virus, serum was taken from mice infected with B/Yamagata, B/201, or B/234 virus and analyzed for antibodies directed against purified B/Yamagata virus. Infection with either B/Yamagata, B/201, or B/234 virus resulted in a significant induction of influenza B/Yamagata virus-specific serum IgG (Fig. 7). Furthermore, although viral lung titers vary significantly among groups of mice infected with these influenza B viruses, B/Yamagata-specific antibody titers appear to be generated to roughly similar levels.
Figure 7.
Influenza B virus-specific antibody titers of sera taken from mice immunized with B/Yamagata, B/201, or B/234 virus. Serum samples were tested from mice immunized with 3 × 105 pfu of the indicated virus or from mock immunized mice. Serum samples were taken from mice 4 wk after immunization and before challenge with wild-type B/Yamagata virus. Samples were tested for their reactivity against sucrose banded B/Yamagata virus. OD405 readings for sera diluted 1:1,000 are shown.
Discussion
Successful live virus vaccine candidates must satisfy the following criteria: growth to high titers in a suitable preparative medium, attenuation in the host, and immunogenicity. In this study, we use influenza virus as a model system to explore the alteration of viral IFN antagonists as a means of creating vaccine strains that satisfy these requirements.
Growth in Embryonated Eggs of Influenza A Viruses.
Our results indicate that alterations in the NS1 ORFs of influenza A viruses affect the growth properties of these viruses in embryonated eggs. Influenza A/delNS1 is a recombinant virus that completely lacks the anti-IFN NS1 protein. This virus grows poorly in embryonated eggs older than 7 days (Fig. 2 A and B). We speculate that this reduction in growth in older eggs is because of maturation of the host innate immune system and the inability of the virus to counteract the increasing IFN response in older embryonated eggs (21). Influenza A/NS1–99 virus encodes a truncated NS1 protein containing 99 N-terminal amino acids of the wild-type protein. NS1–99 demonstrates intermediate growth characteristics in eggs as compared with delNS1 and wild-type PR8 viruses (Fig. 2A), suggesting that the shortened NS1 protein encoded by NS1–99 virus retains partial function. We have not yet determined the precise mechanism by which the NS1 protein counteracts the IFN response of the cell. The NS1 protein of influenza A virus has several reported activities (reviewed in ref. 23), including the inhibition of the IFN induced protein kinase, PKR (24, 25). The influenza A NS1 protein has also been shown to bind to several RNA species including double-stranded RNA (25–27), a potent activator of the IFN response (28). Reduction or loss of one or more of these NS1 activities may contribute to virus attenuation. Despite the reduction in growth of NS1-attenuated influenza A viruses in older eggs, younger eggs have proven to be suitable media for preparative growth of these viruses to titers sufficient for vaccination study.
Attenuation in Mice of Influenza A delNS1 and NS1–99 Viruses.
Another important characteristic for a potential live vaccine is attenuation in the host. When administered to mice, delNS1 and NS1–99 viruses are much less virulent than wild-type influenza A/PR8 virus in BALB/c mice (Table 1). Intranasal inoculation with 1 × 106 pfu of delNS1 does not cause disease (data not shown) or death in infected mice. Furthermore, the LD50 of NS1–99 virus is significantly greater than the LD50 for PR8 virus (i.e., 1 × 103 pfu), because 80% of mice infected with 1 × 106 pfu of NS1–99 virus survived.
Like the immunologically mature embryonated egg, the wild-type mouse represents an IFN competent environment. Without the ability to fully counteract this IFN response, delNS1 and NS1–99 viruses are severely weakened within the mouse host. The argument that alteration of virally encoded IFN antagonists correlates with attenuation is strengthened by recent work on paramyxoviruses (29–31).
Protection of delNS1 and NS1–99 Immunized Mice.
We also wanted to assess the ability of influenza A viruses encoding altered NS1 proteins to induce a protective immune response. We found that although delNS1 and NS1–99 viruses are attenuated in the mouse host, they are still able to induce an immune response capable of providing a significant degree of protection against challenge with a highly lethal dose (5,000 LD50) of wild-type influenza A/PR8 virus. Mice immunized with 1 × 106 pfu of delNS1 or NS1–99 virus gave measurable antibody titers and were 100% protected against lethal challenge. As expected, reduction in the immunizing dose of either delNS1 or NS1–99 virus resulted in lower levels of PR8-specific serum IgG and a decrease in the number of mice protected against wild-type challenge (groups A–D, Fig. 3, and Table 1). The antibody levels in groups B and D do not correlate with protection. However, vaccination with this dose of NS1–99 virus appears to induce better protection than an equivalent dose of delNS1 virus. We plan to investigate whether other NS1 mutants may be more immunogenic and also whether the protection induced by delNS1 and NS1–99 viruses may be improved by a boosting protocol.
Both delNS1 and NS1–99 viruses induced influenza NP peptide-specific CD8 + spleen cells to numbers comparable to wild-type influenza PR8 virus (Fig. 4), indicating that immunization with these NS1-altered viruses results in the stimulation of this cell-mediated component of the immune system. Although cell-mediated immunity may not be sufficient for complete protection, it has been shown to be critical in the clearance of influenza virus from the infected host (32).
Attenuated Influenza B Viruses.
Influenza A and B viral NS1 proteins share little sequence homology. However, like the influenza A NS1 protein, influenza B NS1 is able to inhibit the activation of the IFN-induced protein kinase (PKR) and binds to double-stranded RNA in vitro (33).
We hypothesized that the influenza B NS1 protein acts as an IFN antagonist. We demonstrate here that growth in eggs of influenza B viruses containing altered NS1 proteins (B/234 and B/201 viruses) is inversely proportional to previously reported IFN inducibility (Fig. 6) (20, 21). B/234 and B/201 viruses were also attenuated in BALB/c mice (Table 2), showing dramatic reduction in pfu titers in the mouse lung at 3 days after infection as compared with wild-type B/Yamagata virus. Although attenuated, influenza B/234 and B/201 viruses induced an immunological response in BALB/c mice capable of reducing the infectivity of wild-type B/Yamagata virus (Fig. 7 and Table 2).
We speculate that growth attenuation of B/234 and B/201 viruses is a result of a decrease in the ability of altered NS1 proteins encoded by these viruses to act as IFN antagonists. However, the ability of influenza B viral NS1 to act as an IFN antagonist awaits further functional analysis.
A Vaccine Approach.
To counteract the rapid and efficient induction of an antiviral state by type I IFNs, many viruses encode IFN antagonists (reviewed in ref. 34). The NS1 protein of influenza A virus has been shown to play an important role in this aspect of the replicative cycle of this virus (4). We show that influenza A and B viruses containing alterations in the NS1 protein are attenuated and provide protective immunity against challenge with wild-type virus. We propose that deletion of virally encoded IFN antagonists or mutagenesis of these proteins to reduce activity can be used as a general strategy to construct live viral vaccines that are optimally attenuated and immunogenic.
Acknowledgments
We thank Kiyotake Tobita (Jichi Medical School, Tochigi, Japan) for kindly providing us with the influenza B/Yamagata/1/73 clone 201 and AWBY-234 viruses. The authors also thank Rosalind Polley (University of Bath, Bath, U.K.) for her assistance with HA titration and mouse care and Ana Fernandez-Sesma (Mount Sinai School of Medicine, New York) for her guidance with the ELISA. This work was supported in part by grants to A.G.-S. and P.P. from the National Institutes of Health and by the Austrian National Bank (T.M.).
Abbreviations
- MDCK cells
Madin–Darby canine kidney cells
- MOI
multiplicity of infection
- pfu
plaque-forming unit
- NEP
nuclear export protein
- HA
hemagglutination
- NP
nuclear protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070525997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070525997
References
- 1.Maassab H F, Herlocher M L, Bryant M L. In: Vaccines. Plotkin S A, Orenstein W A, editors. Philadelphia: Saunders; 1999. pp. 909–927. [Google Scholar]
- 2.Gorse G J, Belshe R B. J Clin Microbiol. 1990;28:2539–2550. doi: 10.1128/jcm.28.11.2539-2550.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorse G J, Belshe R B. Scand J Infect Dis. 1991;23:7–17. doi: 10.3109/00365549109023368. [DOI] [PubMed] [Google Scholar]
- 4.García-Sastre A, Egorov A, Matassov D, Brandt S, Levy D E, Durbin J E, Palese P, Muster T. Virology. 1998;252:324–330. doi: 10.1006/viro.1998.9508. [DOI] [PubMed] [Google Scholar]
- 5.Luytjes W, Krystal M, Enami M, Pavin J D, Palese P. Cell. 1989;59:1107–1113. doi: 10.1016/0092-8674(89)90766-6. [DOI] [PubMed] [Google Scholar]
- 6.Tobita K, Tanaka T, Odagiri T, Tashiro M, Feng S Y. Virology. 1990;174:314–319. doi: 10.1016/0042-6822(90)90082-3. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka T, Urabe M, Goto H, Tobita K. Virology. 1984;135:515–523. doi: 10.1016/0042-6822(84)90205-8. [DOI] [PubMed] [Google Scholar]
- 8.Norton G P, Tanaka T, Tobita K, Nakada S, Buonagurio D A, Greenspan D, Krystal M, Palese P. Virology. 1987;156:204–213. doi: 10.1016/0042-6822(87)90399-0. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T, Odagiri T, Tobita K. Arch Virol. 1988;102:173–185. doi: 10.1007/BF01310823. [DOI] [PubMed] [Google Scholar]
- 10.O'Neill R E, Talon J, Palese P. EMBO J. 1998;17:288–296. doi: 10.1093/emboj/17.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, et al. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 12.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou W M, Fiers W. Nat Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 13.Novak M, Moldoveanu Z, Schafer D P, Mestecky J, Compans R W. Vaccine. 1993;11:55–60. doi: 10.1016/0264-410x(93)90339-y. [DOI] [PubMed] [Google Scholar]
- 14.Lu X, Tumpey T M, Morken T, Zaki S R, Cox N J, Katz J M. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Fabio S, Mbawuike I N, Kiyono H, Fujihashi K, Couch R B, McGhee J R. Int Immunol. 1994;6:11–19. doi: 10.1093/intimm/6.1.11. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues M, Li S, Murata K, Rodriguez D, Rodriguez J R, Bacik I, Bennink J R, Yewdell J W, Garcia-Sastre A, Nussenzweig R S, et al. J Immunol. 1994;153:4636–4648. [PubMed] [Google Scholar]
- 17.Diaz M O, Ziemin S, Le Beau M M, Pitha P, Smith S D, Chilcote R R, Rowley J D. Proc Natl Acad Sci USA. 1988;85:5259–5263. doi: 10.1073/pnas.85.14.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mosca J D, Pitha P M. Mol Cell Biol. 1986;6:2279–2283. doi: 10.1128/mcb.6.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekellick M J, Marcus P I. J Interferon Res. 1985;5:651–667. doi: 10.1089/jir.1985.5.651. [DOI] [PubMed] [Google Scholar]
- 20.Sekellick M J, Biggers W J, Marcus P I. In Vitro Cell Dev Biol. 1990;26:997–1003. doi: 10.1007/BF02624475. [DOI] [PubMed] [Google Scholar]
- 21.Morahan P S, Grossberg S E. J Infect Dis. 1970;121:615–623. doi: 10.1093/infdis/121.6.615. [DOI] [PubMed] [Google Scholar]
- 22.Moran T M, Park H, Fernandez-Sesma A, Schulman J L. J Infect Dis. 1999;180:579–585. doi: 10.1086/314952. [DOI] [PubMed] [Google Scholar]
- 23.Krug R M. In: Textbook of Influenza. Nicholson K G, Webster R G, Hay A J, editors. Oxford: Blackwell; 1998. pp. 82–92. [Google Scholar]
- 24.Tan S L, Katze M G. J Interferon Cytokine Res. 1998;18:757–766. doi: 10.1089/jir.1998.18.757. [DOI] [PubMed] [Google Scholar]
- 25.Lu Y, Wambach M, Katze M G, Krug R M. Virology. 1995;214:222–228. doi: 10.1006/viro.1995.9937. [DOI] [PubMed] [Google Scholar]
- 26.Qiu Y, Krug R M. J Virol. 1994;68:2425–2432. doi: 10.1128/jvi.68.4.2425-2432.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu Y, Nemeroff M, Krug R M. RNA. 1995;1:304–316. [PMC free article] [PubMed] [Google Scholar]
- 28.Jacobs B L, Langland J O. Virology. 1996;219:339–349. doi: 10.1006/viro.1996.0259. [DOI] [PubMed] [Google Scholar]
- 29.Garcin D, Latorre P, Kolakofsky D. J Virol. 1999;73:6559–6565. doi: 10.1128/jvi.73.8.6559-6565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Didcock L, Young D F, Goodbourn S, Randall R E. J Virol. 1999;73:3125–3133. doi: 10.1128/jvi.73.4.3125-3133.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Didcock L, Young D F, Goodbourn S, Randall R E. J Virol. 1999;73:9928–9933. doi: 10.1128/jvi.73.12.9928-9933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mackenzie C D, Taylor P M, Askonas B A. Immunology. 1989;67:375–381. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang W, Krug R M. Virology. 1996;223:41–50. doi: 10.1006/viro.1996.0453. [DOI] [PubMed] [Google Scholar]
- 34.Stark G R, Kerr I M, Williams B R, Silverman R H, Schreiber R D. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 35.Baez M, Taussig R, Zazra J J, Young J F, Palese P, Reisfeld A, Skalka A M. Nucleic Acids Res. 1980;8:5845–5858. doi: 10.1093/nar/8.23.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]