Abstract
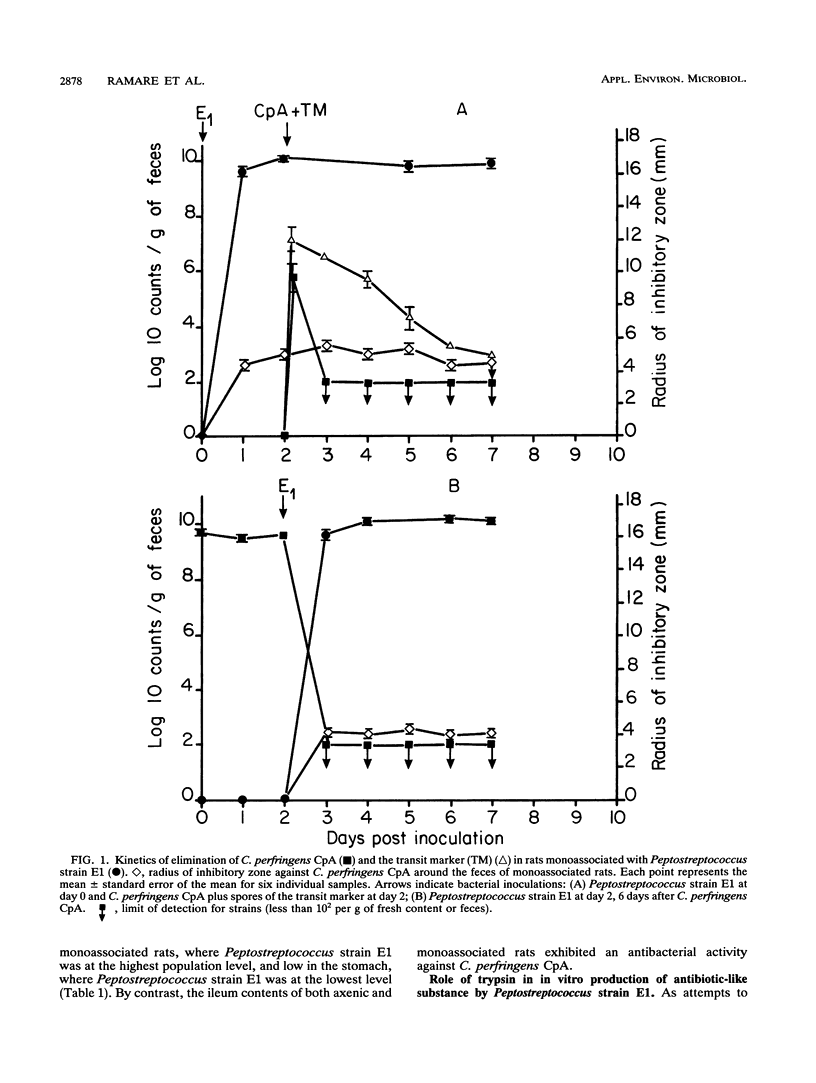
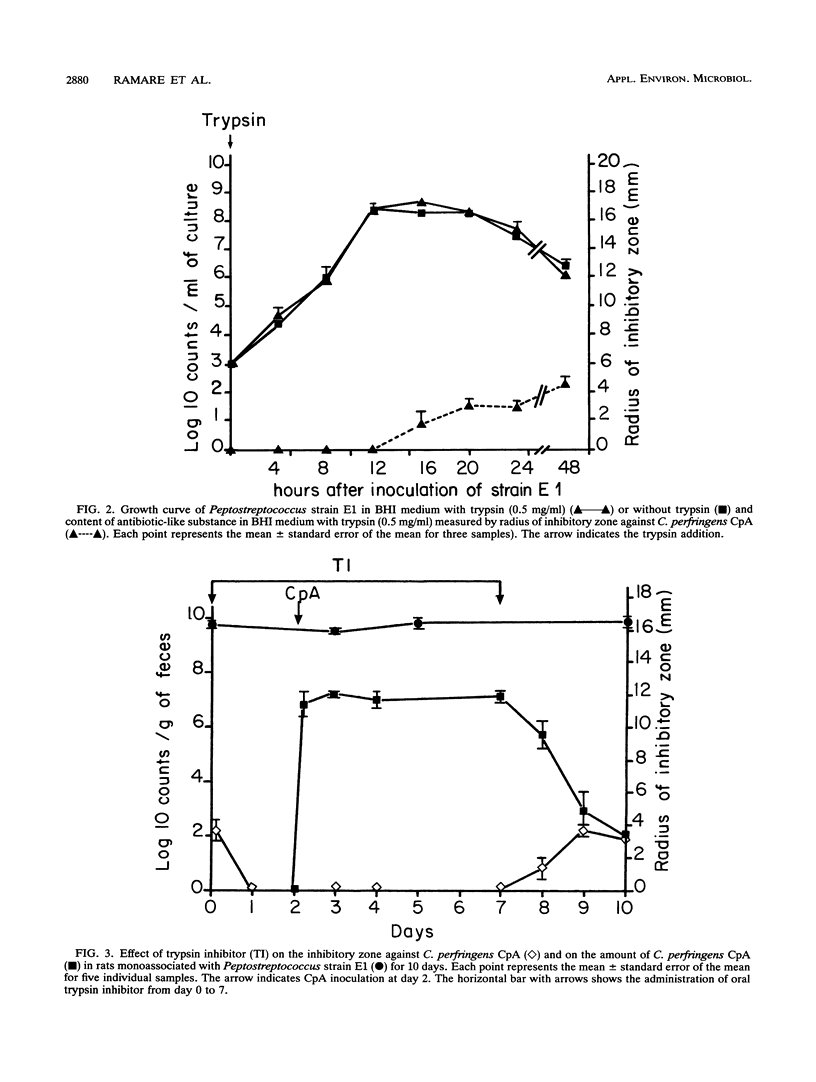
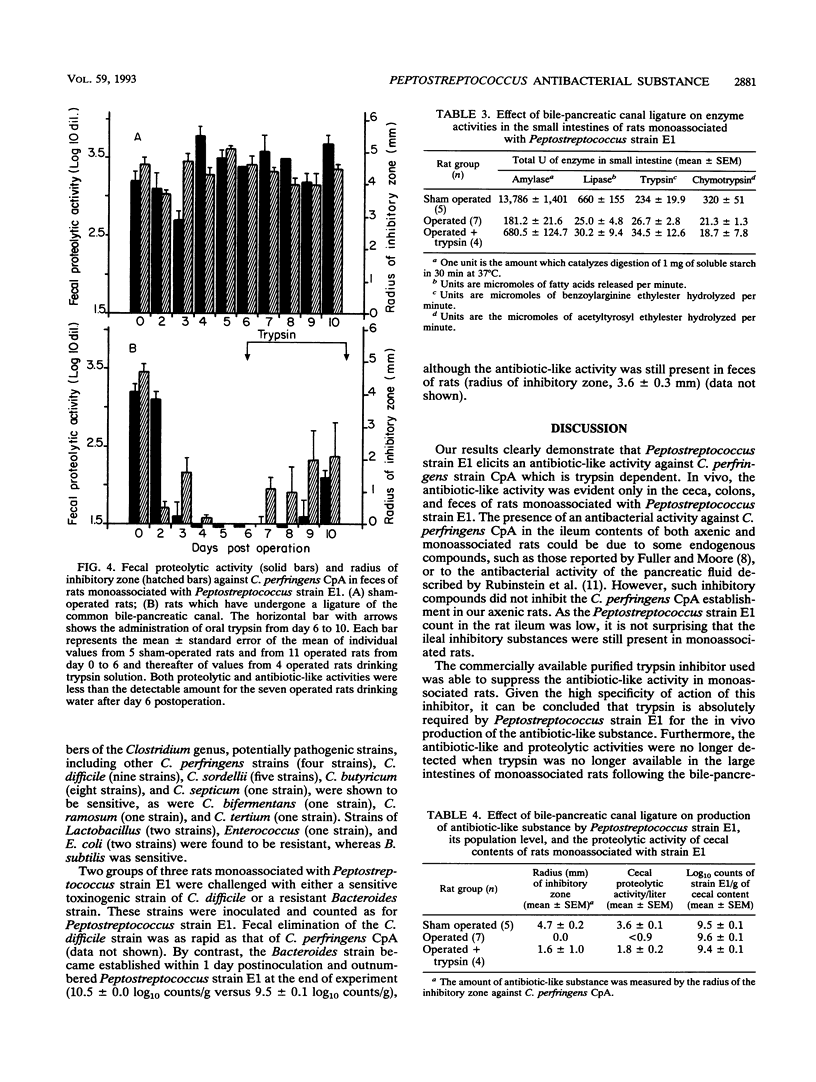
An antibacterial substance appeared within 1 day in feces of gnotobiotic rats harboring a human intestinal Peptostreptococcus strain. It disappeared when the rat bile-pancreatic duct was ligatured or when the rats ingested a trypsin inhibitor. Anaerobic cultures of the Peptostreptococcus strain in a medium supplemented with trypsin also exhibited an antibacterial activity, which was also inhibited by the trypsin inhibitor. In vitro the antibacterial substance from both feces and culture medium was active against several gram-positive bacteria, including other Peptostreptococcus spp., potentially pathogenic Clostridium spp. such as C. perfringens, C. difficile, C. butyricum, C. septicum, and C. sordellii, Eubacterium spp., Bifidobacterium spp., and Bacillus spp. Whatever the order of inoculation of the strains, a sensitive strain of C. perfringens was eliminated within 1 day from the intestine of rats monoassociated with the Peptostreptococcus strain. These findings demonstrate for the first time that very potent antibacterial substances can be produced through a mechanism involving intestinal bacteria and exocrine pancreatic secretions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corring T., Moreau C., Ducluzeau R. Comparative apparent digestibility of casein in holoxenic, axenic, and Clostridium bifermentans monoassociated rats. Am J Clin Nutr. 1979 Jun;32(6):1231–1237. doi: 10.1093/ajcn/32.6.1231. [DOI] [PubMed] [Google Scholar]
- Corring T., Saucier R. Sécrétion pancréatique sur porc fistulé. Adaptation a la teneur en protéines du régime. Ann Biol Anim Biochim Biophys. 1972;12(2):233–241. [PubMed] [Google Scholar]
- Corthier G., Muller M. C., Elmer G. W., Lucas F., Dubos-Ramaré F. Interrelationships between digestive proteolytic activities and production and quantitation of toxins in pseudomembranous colitis induced by Clostridium difficile in gnotobiotic mice. Infect Immun. 1989 Dec;57(12):3922–3927. doi: 10.1128/iai.57.12.3922-3927.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducluzeau R., Bellier M., Raibaud P. Transit Digestif de Divers Inoculums Bactériens Introduits "Per Os" Chez des Souris Axéniques et "Holoxéniques" (Conventionnelles): Effet Antagoniste de la Microflore du Tractus Gastro-Intestinal. Zentralbl Bakteriol Orig. 1970 May;213(4):533–548. [PubMed] [Google Scholar]
- Ducluzeau R., Dubos F., Raibaud P., Abrams G. D. Inhibition of Clostridium perfringens by an antibiotic substance produced by Bacillus licheniformis in the digestive tract of gnotobiotic mice: effect on other bacteria from the digestive tract. Antimicrob Agents Chemother. 1976 Jan;9(1):20–25. doi: 10.1128/aac.9.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval-Iflah Y., Raibaud P., Rousseau M. Antagonisms among isogenic strains of Escherichia coli in the digestive tracts of gnotobiotic mice. Infect Immun. 1981 Dec;34(3):957–969. doi: 10.1128/iai.34.3.957-969.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R., Moore J. H. The inhibition of the growth of Clostridium welchii by lipids isolated from the contents of the small intestine of the pig. J Gen Microbiol. 1967 Jan;46(1):23–41. doi: 10.1099/00221287-46-1-23. [DOI] [PubMed] [Google Scholar]
- REBOUD J. P., BEN ABDEL JLIL A., DESNUELLE P. [Variations in the enzyme content of the rat pancreas as a function of the composition of the diet]. Biochim Biophys Acta. 1962 Apr 9;58:326–337. doi: 10.1016/0006-3002(62)91016-8. [DOI] [PubMed] [Google Scholar]
- Rathelot J., Julien R., Canioni P., Coeroli C., Sarda L. Studies on the effect of bile salt and colipase on enzymatic lipolysis. Improved method for the determination of pancreatic lipase and colipase. Biochimie. 1975;57(10):1117–1122. doi: 10.1016/s0300-9084(76)80572-x. [DOI] [PubMed] [Google Scholar]
- Rubinstein E., Mark Z., Haspel J., Ben-Ari G., Dreznik Z., Mirelman D., Tadmor A. Antibacterial activity of the pancreatic fluid. Gastroenterology. 1985 Apr;88(4):927–932. doi: 10.1016/s0016-5085(85)80009-3. [DOI] [PubMed] [Google Scholar]
- Yurdusev N., Ladire M., Ducluzeau R., Raibaud P. Antagonism exerted by an association of a Bacteroides thetaiotaomicron strain and a Fusobacterium necrogenes strain against Clostridium perfringens in gnotobiotic mice and in fecal suspensions incubated in vitro. Infect Immun. 1989 Mar;57(3):724–731. doi: 10.1128/iai.57.3.724-731.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


