Abstract
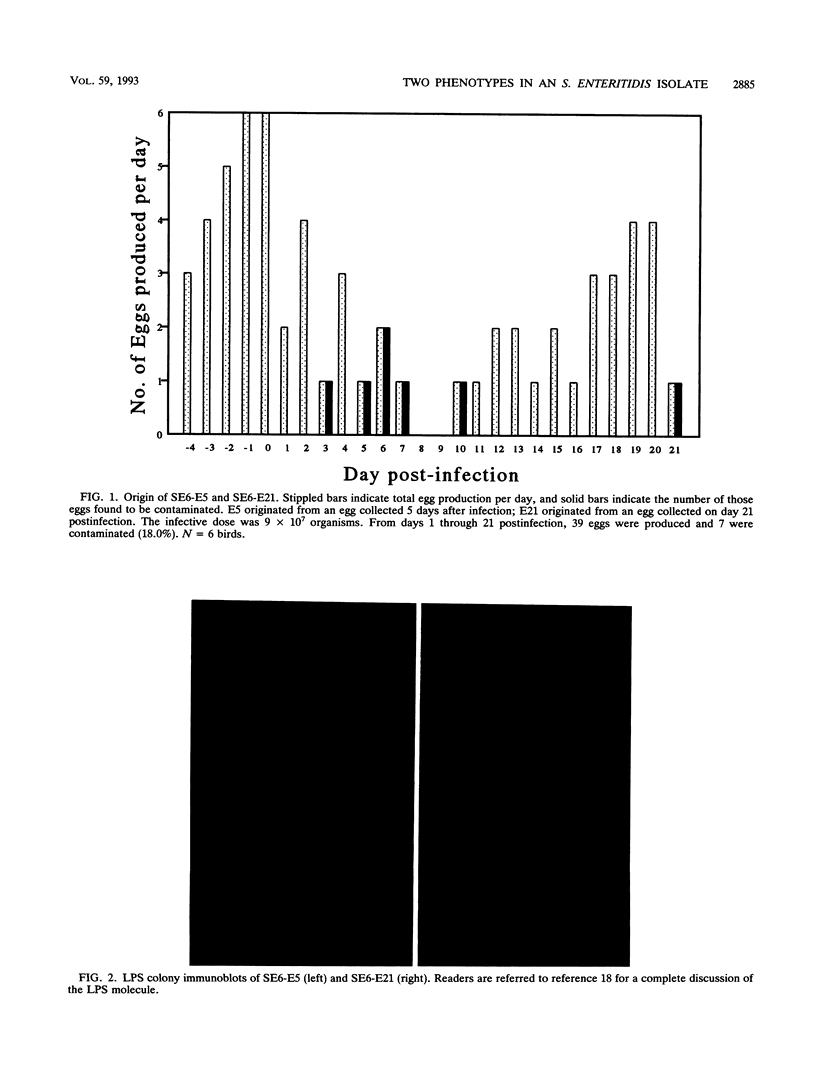
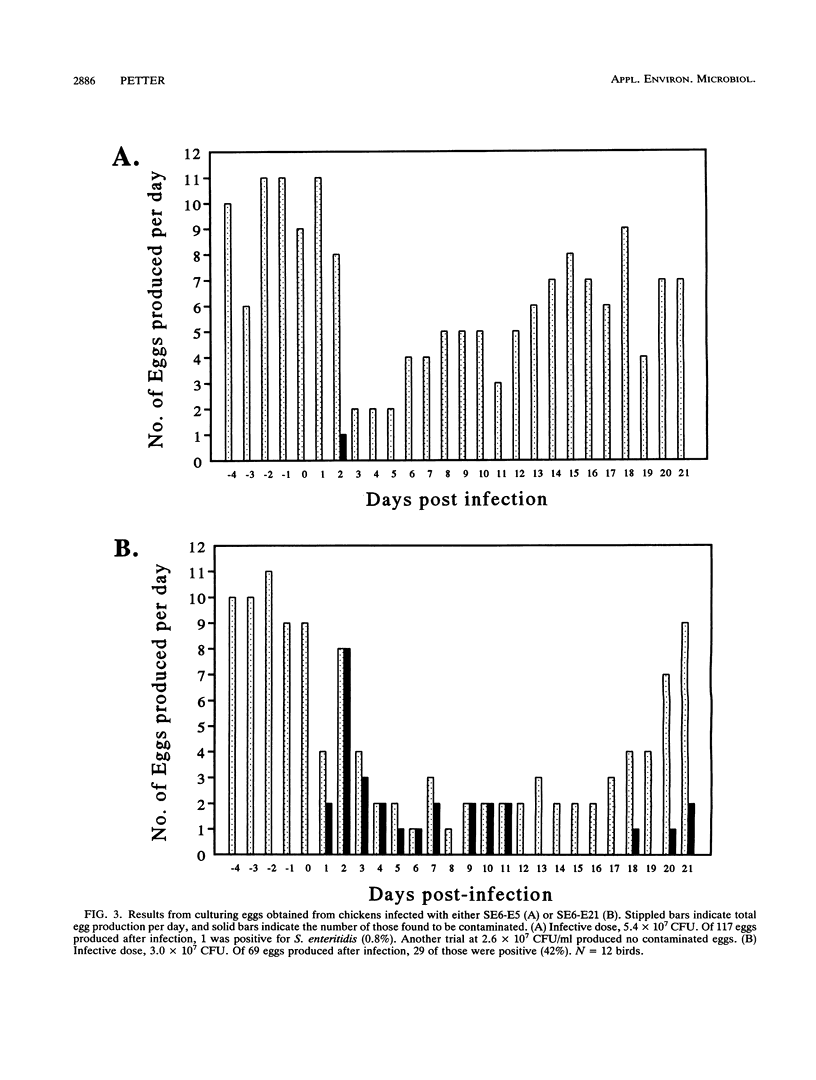
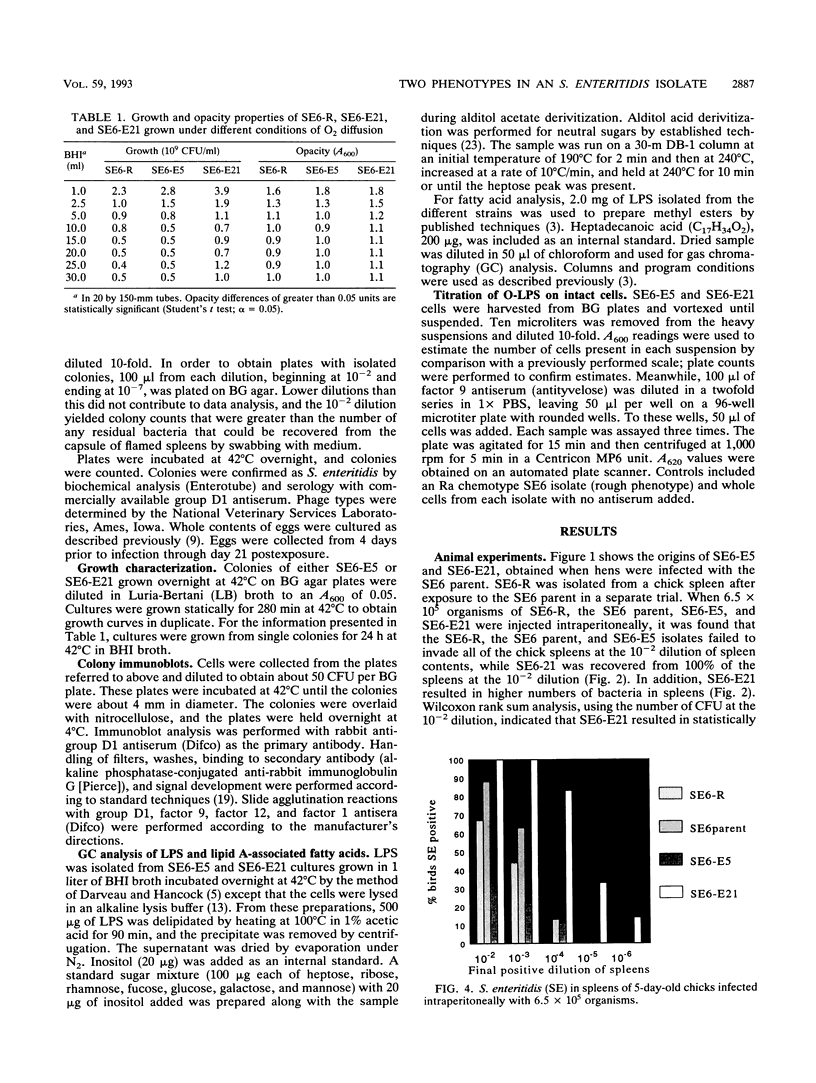
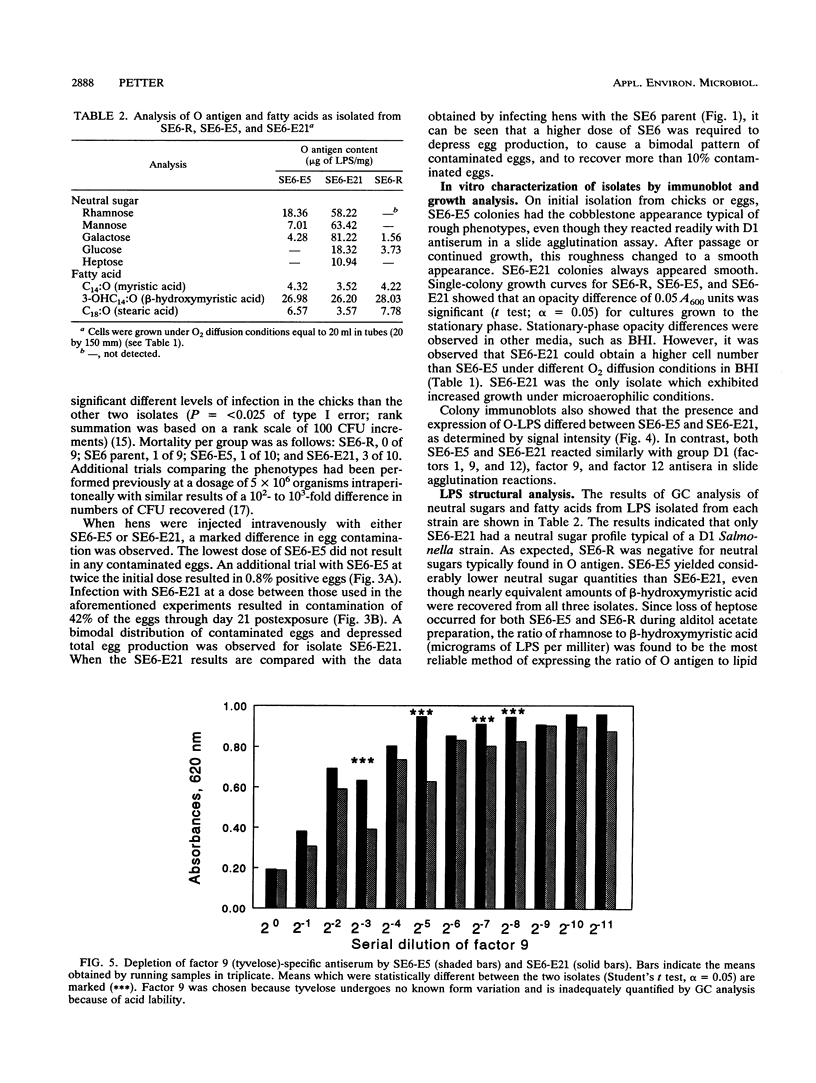
Salmonella enteritidis isolates were obtained from eggs after infection of Leghorn hens with a parent isolate (SE6) known to only infrequently contaminate eggs. Isolates from eggs exhibited two phenotypes that were subtly different. One phenotype was typically smooth, while the other was transiently rough. Both sets of isolates were phage type 13A and positive for D1 epitopes. Immunoblot analysis of entire colonies and gas chromatographic analysis of purified lipopolysaccharide revealed that the phenotypic difference between isolates was due to a quantitative difference in O antigen and possibly a qualitative difference in the lipid A core region. In addition, the two isolates had different opacity properties when examined at 600 nm. When the two isolates were used to inject egg-laying hens, a significant difference in the ability to contaminate eggs was detected.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey M. J., Koronakis V., Schmoll T., Hughes C. Escherichia coli HlyT protein, a transcriptional activator of haemolysin synthesis and secretion, is encoded by the rfaH (sfrB) locus required for expression of sex factor and lipopolysaccharide genes. Mol Microbiol. 1992 Apr;6(8):1003–1012. doi: 10.1111/j.1365-2958.1992.tb02166.x. [DOI] [PubMed] [Google Scholar]
- Bhat U. R., Mayer H., Yokota A., Hollingsworth R. I., Carlson R. W. Occurrence of lipid A variants with 27-hydroxyoctacosanoic acid in lipopolysaccharides from members of the family Rhizobiaceae. J Bacteriol. 1991 Apr;173(7):2155–2159. doi: 10.1128/jb.173.7.2155-2159.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H., Row B., Threlfall E. J., Ward L. R. Conversion of Salmonella enteritidis phage type 4 to phage type 7 involves loss of lipopolysaccharide with concomitant loss of virulence. FEMS Microbiol Lett. 1989 Jul 1;51(1):37–40. doi: 10.1016/0378-1097(89)90073-6. [DOI] [PubMed] [Google Scholar]
- Darveau R. P., Hancock R. E. Procedure for isolation of bacterial lipopolysaccharides from both smooth and rough Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983 Aug;155(2):831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B. Regulation of bacterial oxidative stress genes. Annu Rev Genet. 1991;25:315–337. doi: 10.1146/annurev.ge.25.120191.001531. [DOI] [PubMed] [Google Scholar]
- Ebel E. D., David M. J., Mason J. Occurrence of Salmonella enteritidis in the U.S. commercial egg industry: report on a national spent hen survey. Avian Dis. 1992 Jul-Sep;36(3):646–654. [PubMed] [Google Scholar]
- Erwin A. L., Munford R. S. Deacylation of structurally diverse lipopolysaccharides by human acyloxyacyl hydrolase. J Biol Chem. 1990 Sep 25;265(27):16444–16449. [PubMed] [Google Scholar]
- Grossman N., Schmetz M. A., Foulds J., Klima E. N., Jimenez-Lucho V. E., Leive L. L., Joiner K. A., Jiminez V. Lipopolysaccharide size and distribution determine serum resistance in Salmonella montevideo. J Bacteriol. 1987 Feb;169(2):856–863. doi: 10.1128/jb.169.2.856-863.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issartel J. P., Koronakis V., Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature. 1991 Jun 27;351(6329):759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- Kido N., Ohta M., Kato N. Detection of lipopolysaccharides by ethidium bromide staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Bacteriol. 1990 Feb;172(2):1145–1147. doi: 10.1128/jb.172.2.1145-1147.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. D. Salmonella enteritidis infection in broiler chickens. Vet Rec. 1988 Feb 27;122(9):214–214. doi: 10.1136/vr.122.9.214-b. [DOI] [PubMed] [Google Scholar]
- St Louis M. E., Morse D. L., Potter M. E., DeMelfi T. M., Guzewich J. J., Tauxe R. V., Blake P. A. The emergence of grade A eggs as a major source of Salmonella enteritidis infections. New implications for the control of salmonellosis. JAMA. 1988 Apr 8;259(14):2103–2107. [PubMed] [Google Scholar]
- Ward L. R., de Sa J. D., Rowe B. A phage-typing scheme for Salmonella enteritidis. Epidemiol Infect. 1987 Oct;99(2):291–294. doi: 10.1017/s0950268800067765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambrano M. M., Siegele D. A., Almirón M., Tormo A., Kolter R. Microbial competition: Escherichia coli mutants that take over stationary phase cultures. Science. 1993 Mar 19;259(5102):1757–1760. doi: 10.1126/science.7681219. [DOI] [PubMed] [Google Scholar]



