Abstract
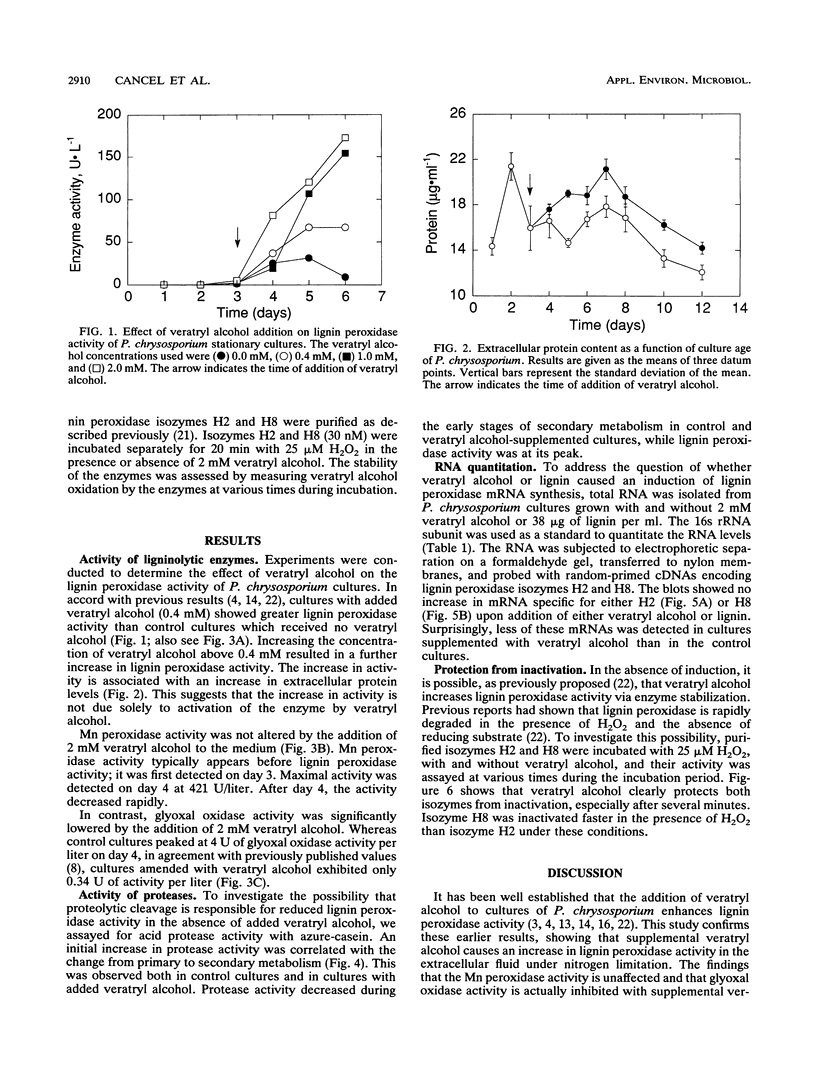
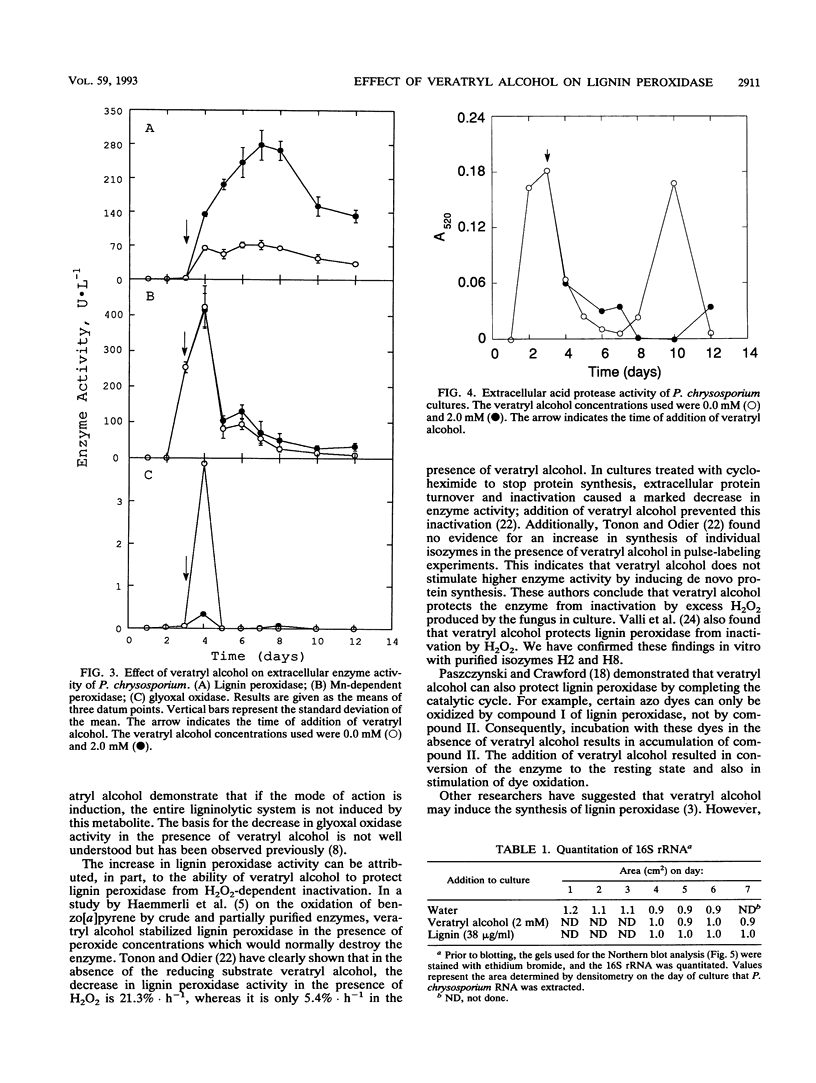
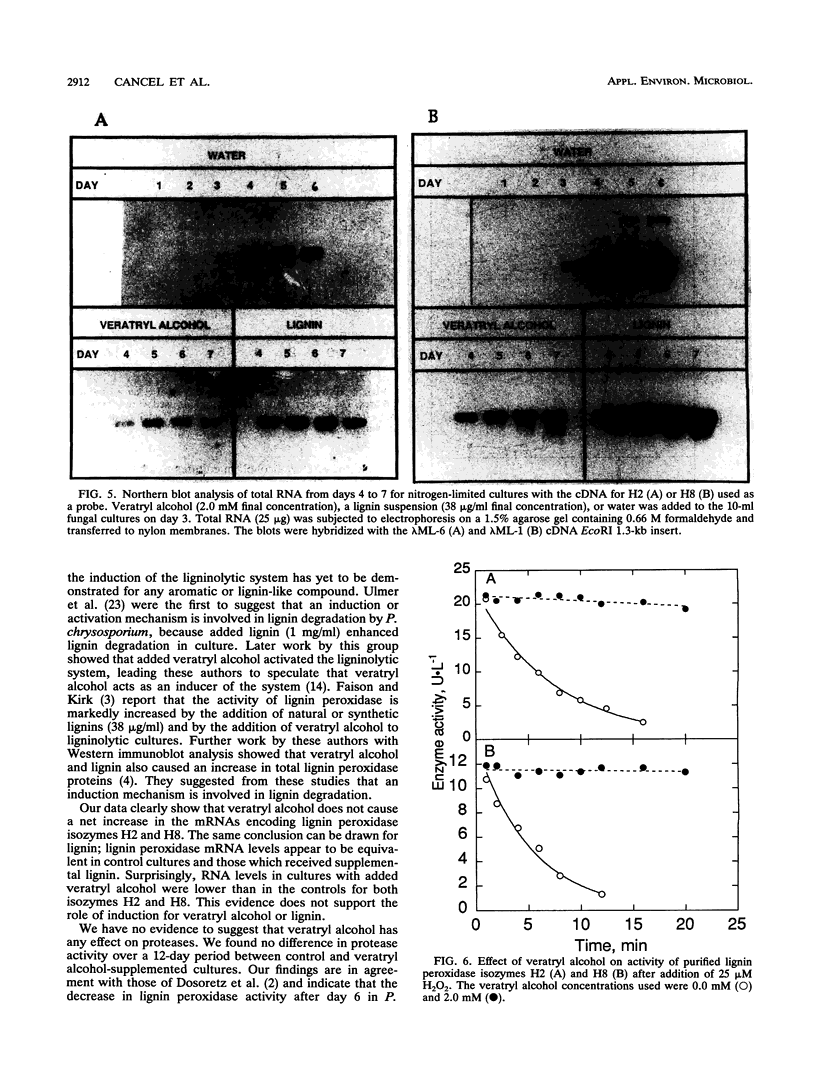
Phanerochaete chrysosporium is a white rot fungus which secretes a family of lignin-degrading enzymes under nutrient limitation. In this work, we investigated the roles of veratryl alcohol and lignin in the ligninolytic system of P. chrysosporium BKM-F-1767 cultures grown under nitrogen-limited conditions. Cultures supplemented with 0.4 to 2 mM veratryl alcohol showed increased lignin peroxidase activity. Addition of veratryl alcohol had no effect on Mn-dependent peroxidase activity and inhibited glyoxal oxidase activity. Azure-casein analysis of acidic proteases in the extracellular fluid showed that protease activity decreased during the early stages of secondary metabolism while lignin peroxidase activity was at its peak, suggesting that proteolysis was not involved in the regulation of lignin peroxidase activity during early secondary metabolism. In cultures supplemented with lignin or veratryl alcohol, no induction of mRNA coding for lignin peroxidase H2 or H8 was observed. Veratryl alcohol protected lignin peroxidase isozymes H2 and H8 from inactivation by H2O2. We conclude that veratryl alcohol acts as a stabilizer of lignin peroxidase activity and not as an inducer of lignin peroxidase synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosoretz C. G., Chen H. C., Grethlein H. E. Effect of Environmental Conditions on Extracellular Protease Activity in Lignolytic Cultures of Phanerochaete chrysosporium. Appl Environ Microbiol. 1990 Feb;56(2):395–400. doi: 10.1128/aem.56.2.395-400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K. Factors Involved in the Regulation of a Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1985 Feb;49(2):299–304. doi: 10.1128/aem.49.2.299-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faison B. D., Kirk T. K., Farrell R. L. Role of Veratryl Alcohol in Regulating Ligninase Activity in Phanerochaete chrysosporium. Appl Environ Microbiol. 1986 Aug;52(2):251–254. doi: 10.1128/aem.52.2.251-254.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerli S. D., Leisola M. S., Sanglard D., Fiechter A. Oxidation of benzo(a)pyrene by extracellular ligninases of Phanerochaete chrysosporium. Veratryl alcohol and stability of ligninase. J Biol Chem. 1986 May 25;261(15):6900–6903. [PubMed] [Google Scholar]
- Hammel K. E., Tien M., Kalyanaraman B., Kirk T. K. Mechanism of oxidative C alpha-C beta cleavage of a lignin model dimer by Phanerochaete chrysosporium ligninase. Stoichiometry and involvement of free radicals. J Biol Chem. 1985 Jul 15;260(14):8348–8353. [PubMed] [Google Scholar]
- Kirk T. K., Farrell R. L. Enzymatic "combustion": the microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- Kirk T. K., Tien M., Kersten P. J., Mozuch M. D., Kalyanaraman B. Ligninase of Phanerochaete chrysosporium. Mechanism of its degradation of the non-phenolic arylglycerol beta-aryl ether substructure of lignin. Biochem J. 1986 May 15;236(1):279–287. doi: 10.1042/bj2360279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Orth A. B., Denny M., Tien M. Overproduction of lignin-degrading enzymes by an isolate of Phanerochaete chrysosporium. Appl Environ Microbiol. 1991 Sep;57(9):2591–2596. doi: 10.1128/aem.57.9.2591-2596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszczynski A., Crawford R. L. Degradation of azo compounds by ligninase from Phanerochaete chrysosporium: involvement of veratryl alcohol. Biochem Biophys Res Commun. 1991 Aug 15;178(3):1056–1063. doi: 10.1016/0006-291x(91)90999-n. [DOI] [PubMed] [Google Scholar]
- Pease E. A., Andrawis A., Tien M. Manganese-dependent peroxidase from Phanerochaete chrysosporium. Primary structure deduced from cDNA sequence. J Biol Chem. 1989 Aug 15;264(23):13531–13535. [PubMed] [Google Scholar]
- Tien M., Kirk T. K. Lignin-Degrading Enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science. 1983 Aug 12;221(4611):661–663. doi: 10.1126/science.221.4611.661. [DOI] [PubMed] [Google Scholar]
- Tonon F., Odier E. Influence of Veratryl Alcohol and Hydrogen Peroxide on Ligninase Activity and Ligninase Production by Phanerochaete chrysosporium. Appl Environ Microbiol. 1988 Feb;54(2):466–472. doi: 10.1128/aem.54.2.466-472.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valli K., Wariishi H., Gold M. H. Oxidation of monomethoxylated aromatic compounds by lignin peroxidase: role of veratryl alcohol in lignin biodegradation. Biochemistry. 1990 Sep 18;29(37):8535–8539. doi: 10.1021/bi00489a005. [DOI] [PubMed] [Google Scholar]
- Wariishi H., Valli K., Gold M. H. In vitro depolymerization of lignin by manganese peroxidase of Phanerochaete chrysosporium. Biochem Biophys Res Commun. 1991 Apr 15;176(1):269–275. doi: 10.1016/0006-291x(91)90919-x. [DOI] [PubMed] [Google Scholar]
- Zemper E. D., Black S. H. Morphology of freeze-etched Treponema refringens (Nichols). Arch Microbiol. 1978 Jun 26;117(3):227–238. doi: 10.1007/BF00738540. [DOI] [PubMed] [Google Scholar]




