Abstract
NADP(+)-dependent D-threonine dehydrogenase (EC 1.1.1.-), which catalyzes the oxidation of the 3-hydroxyl group of D-threonine, was purified to homogeneity from a crude extract of Pseudomonas cruciviae IFO 12047. The enzyme had a molecular mass of about 60,000 Da and consisted of two identical subunits. In addition to D-threonine, D-threo-3-phenylserine, D-threo-3-thienylserine, and D-threo-3-hydroxynorvaline were also substrates. However, the other isomers of threonine and 3-phenylserine were inert. The enzyme showed maximal activity at pH 10.5 for the oxidation of D-threonine. The enzyme required NADP+. NAD+ showed only slight activity. The enzyme was not inhibited by EDTA, o-phenanthroline, alpha,alpha'-dipyridyl, HgCl2, or p-chloromercuribenzoate but was inhibited by tartronate, malonate, pyruvate, and DL-2-hydroxybutyrate. The inhibition by these organic acids was competitive against D-threonine. Initial-velocity and product inhibition studies suggested that the oxidation proceeded through a sequential ordered Bi Bi mechanism. The Michaelis constants for D-threonine and NADP+ were 13 and 0.12 mM, respectively.
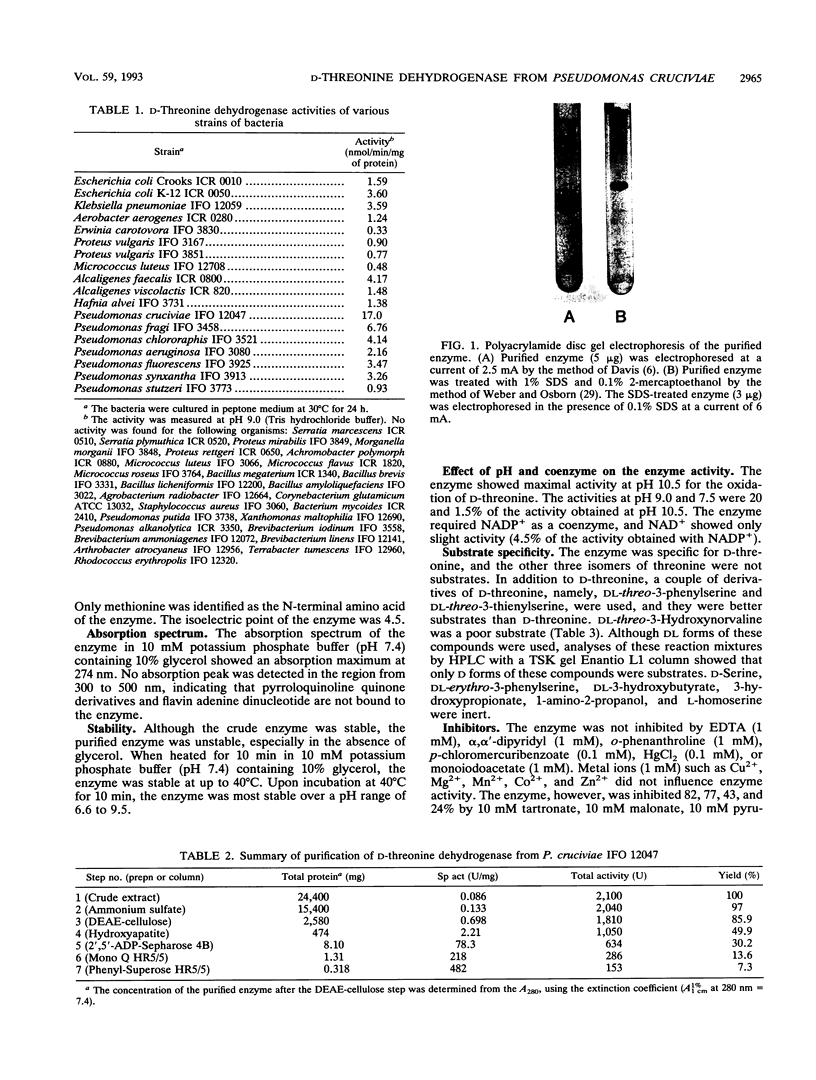
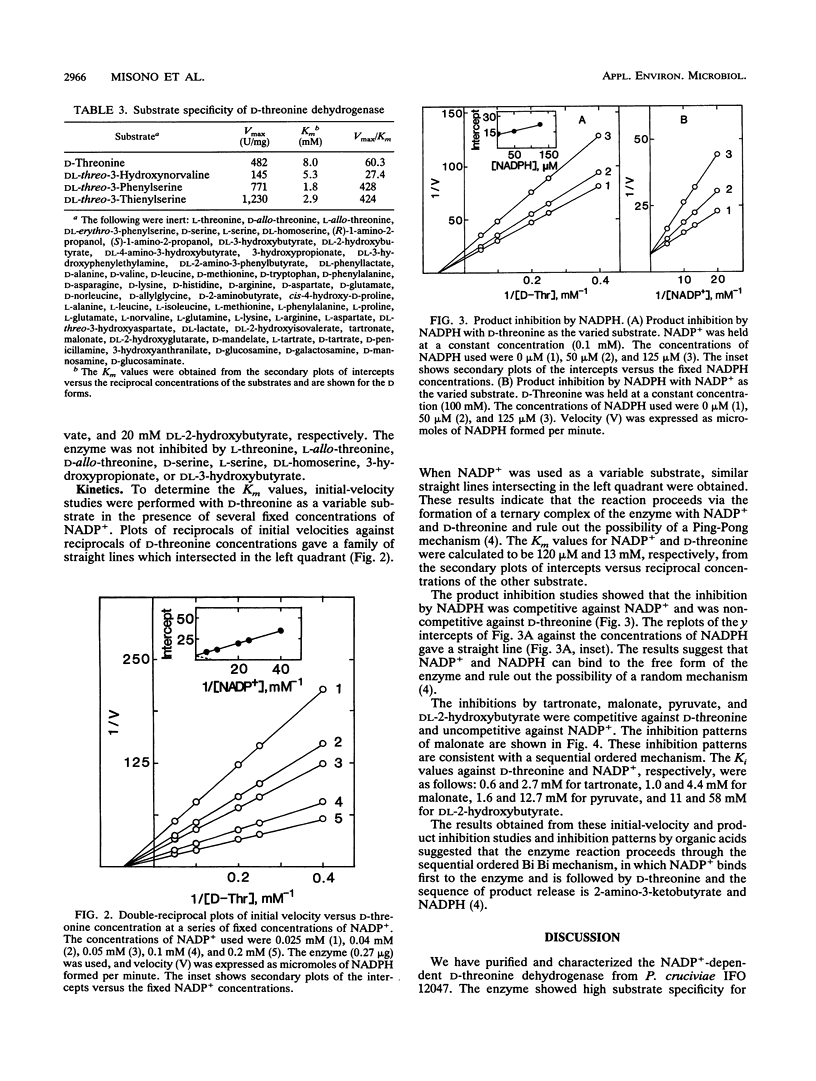
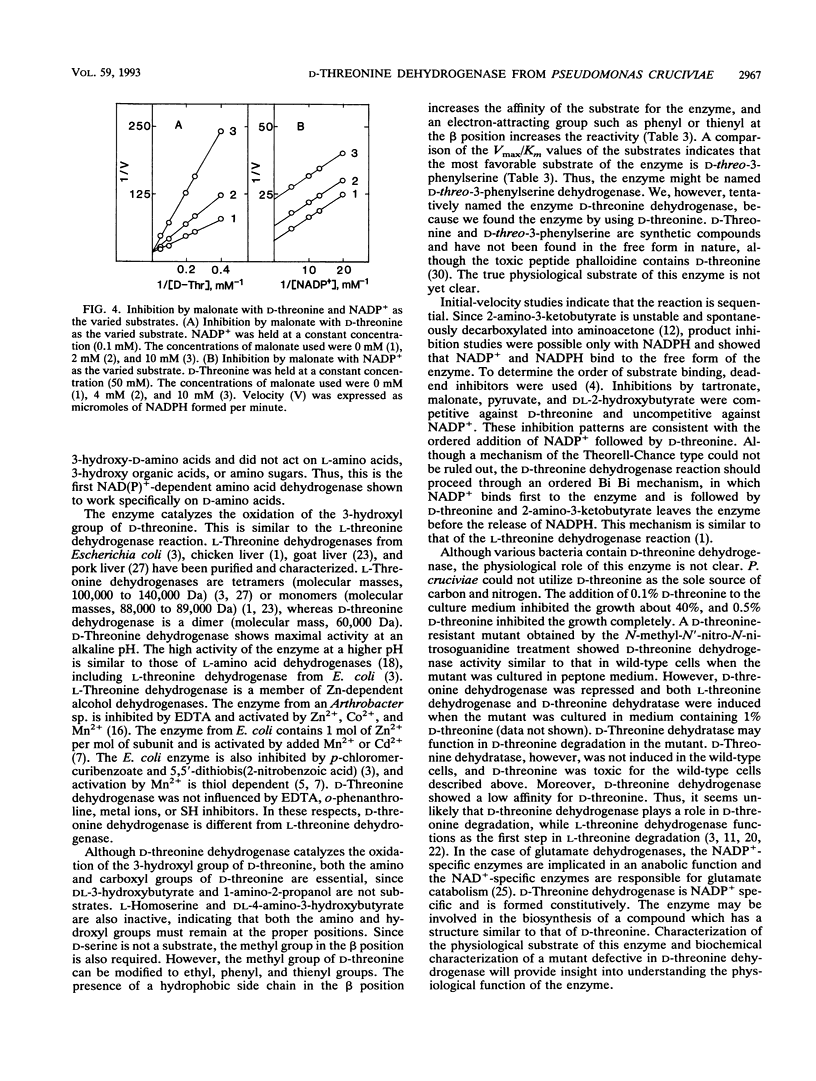
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama Y., Motokawa Y. L-Threonine dehydrogenase of chicken liver. Purification, characterization, and physiological significance. J Biol Chem. 1981 Dec 10;256(23):12367–12373. [PubMed] [Google Scholar]
- Bater A. J., Venables W. A. The characterisation of inducible dehydrogenases specific for the oxidation of D-alanine, allohydroxy-D-proline, choline and sarcosine as peripheral membrane proteins in Pseudomonas aeruginosa. Biochim Biophys Acta. 1977 Jul 14;468(2):209–226. doi: 10.1016/0005-2736(77)90115-8. [DOI] [PubMed] [Google Scholar]
- Boylan S. A., Dekker E. E. L-threonine dehydrogenase. Purification and properties of the homogeneous enzyme from Escherichia coli K-12. J Biol Chem. 1981 Feb 25;256(4):1809–1815. [PubMed] [Google Scholar]
- Craig P. A., Dekker E. E. L-threonine dehydrogenase from Escherichia coli K-12: thiol-dependent activation by Mn2+. Biochemistry. 1986 Apr 22;25(8):1870–1876. doi: 10.1021/bi00356a005. [DOI] [PubMed] [Google Scholar]
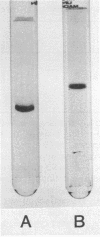
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Epperly B. R., Dekker E. E. L-threonine dehydrogenase from Escherichia coli. Identification of an active site cysteine residue and metal ion studies. J Biol Chem. 1991 Apr 5;266(10):6086–6092. [PubMed] [Google Scholar]
- Franklin F. C., Venables W. A. Biochemical, genetic, and regulatory studies of alanine catabolism in Escherichia coli K12. Mol Gen Genet. 1976 Dec 8;149(2):229–237. doi: 10.1007/BF00332894. [DOI] [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- Hummel W., Kula M. R. Dehydrogenases for the synthesis of chiral compounds. Eur J Biochem. 1989 Sep 1;184(1):1–13. doi: 10.1111/j.1432-1033.1989.tb14983.x. [DOI] [PubMed] [Google Scholar]
- Komatsubara S., Murata K., Kisumi M., Chibata I. Threonine degradation by Serratia marcescens. J Bacteriol. 1978 Aug;135(2):318–323. doi: 10.1128/jb.135.2.318-323.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Magor A. M., Venables W. A. Solubilization, purification and characterization of D-alanine dehydrogenase from Pseudomonas aeruginosa and effects of solubilization on its properties. Biochimie. 1987 Jan;69(1):63–69. doi: 10.1016/0300-9084(87)90272-0. [DOI] [PubMed] [Google Scholar]
- Marshall V. P., Sokatch J. R. Oxidation of D-amino acids by a particulate enzyme from Pseudomonas aeruginosa. J Bacteriol. 1968 Apr;95(4):1419–1424. doi: 10.1128/jb.95.4.1419-1424.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima T., Soda K. Biochemistry and biotechnology of amino acid dehydrogenases. Adv Biochem Eng Biotechnol. 1990;42:187–209. doi: 10.1007/BFb0000734. [DOI] [PubMed] [Google Scholar]
- Olsiewski P. J., Kaczorowski G. J., Walsh C. Purification and properties of D-amino acid dehydrogenase, an inducible membrane-bound iron-sulfur flavoenzyme from Escherichia coli B. J Biol Chem. 1980 May 25;255(10):4487–4494. [PubMed] [Google Scholar]
- Potter R., Kapoor V., Newman E. B. Role of threonine dehydrogenase in Escherichia coli threonine degradation. J Bacteriol. 1977 Nov;132(2):385–391. doi: 10.1128/jb.132.2.385-391.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raunio R. P., Jenkins W. T. D-alanine oxidase form Escherichia coli: localization and induction by L-alanine. J Bacteriol. 1973 Aug;115(2):560–566. doi: 10.1128/jb.115.2.560-566.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnikar P. D., Somerville R. L. Genetic characterization of a highly efficient alternate pathway of serine biosynthesis in Escherichia coli. J Bacteriol. 1987 Jun;169(6):2611–2617. doi: 10.1128/jb.169.6.2611-2617.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray M., Ray S. L-Threonine dehydrogenase from goat liver. Feedback inhibition by methylglyoxal. J Biol Chem. 1985 May 25;260(10):5913–5918. [PubMed] [Google Scholar]
- Scopes R. K. Measurement of protein by spectrophotometry at 205 nm. Anal Biochem. 1974 May;59(1):277–282. doi: 10.1016/0003-2697(74)90034-7. [DOI] [PubMed] [Google Scholar]
- Tressel T., Thompson R., Zieske L. R., Menendez M. I., Davis L. Interaction between L-threonine dehydrogenase and aminoacetone synthetase and mechanism of aminoacetone production. J Biol Chem. 1986 Dec 15;261(35):16428–16437. [PubMed] [Google Scholar]
- Tsukada K. D-amino acid dehydrogenases of Pseudomonas fluorescens. J Biol Chem. 1966 Oct 10;241(19):4522–4528. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wild J., Walczak W., Krajewska-Grynkiewicz K., Klopotowski T. D-amino acid dehydrogenase: the enzyme of the first step of D-histidine and D-methionine racemization in Salmonella typhimurium. Mol Gen Genet. 1974;128(2):131–146. doi: 10.1007/BF02654486. [DOI] [PubMed] [Google Scholar]