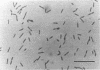
Abstract
A microscopically pure enrichment culture of a gram-negative anaerobic bacterium, in the present article referred to as PER-K23, was isolated from an anaerobic packed-bed column in which tetrachloroethene (PCE) was reductively transformed to ethane via trichloroethene (TCE), cis-1,2-dichloroethene (cis-1,2-DCE), chloroethene, and ethene. PER-K23 catalyzes the dechlorination of PCE via TCE to cis-1,2-DCE and couples this reductive dechlorination to growth. H2 and formate were the only electron donors that supported growth with PCE or TCE as an electron acceptor. The culture did not grow in the absence of PCE or TCE. Neither O2, NO3-, NO2-, SO4(2-), SO3(2-), S2O3(2-), S, nor CO2 could replace PCE or TCE as an electron acceptor with H2 as an electron donor. Also, organic electron acceptors such as acetoin, acetol, dimethyl sulfoxide, fumarate, and trimethylamine N-oxide and chlorinated ethanes, DCEs, and chloroethene were not utilized. PER-K23 was not able to grow fermentatively on any of the organic compounds tested. Transferring the culture to a rich medium revealed that a contaminant was still present. Dechlorination was optimal between pH 6.8 and 7.6 and a temperature of 25 to 35 degrees C. H2 consumption was paralleled by chloride production, PCE degradation, cis-1,2-DCE formation, and growth of PER-K23. Electron balances showed that all electrons derived from H2 or formate consumption were recovered in dechlorination products and biomass. Exponential growth could be achieved only in gently shaken cultures.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


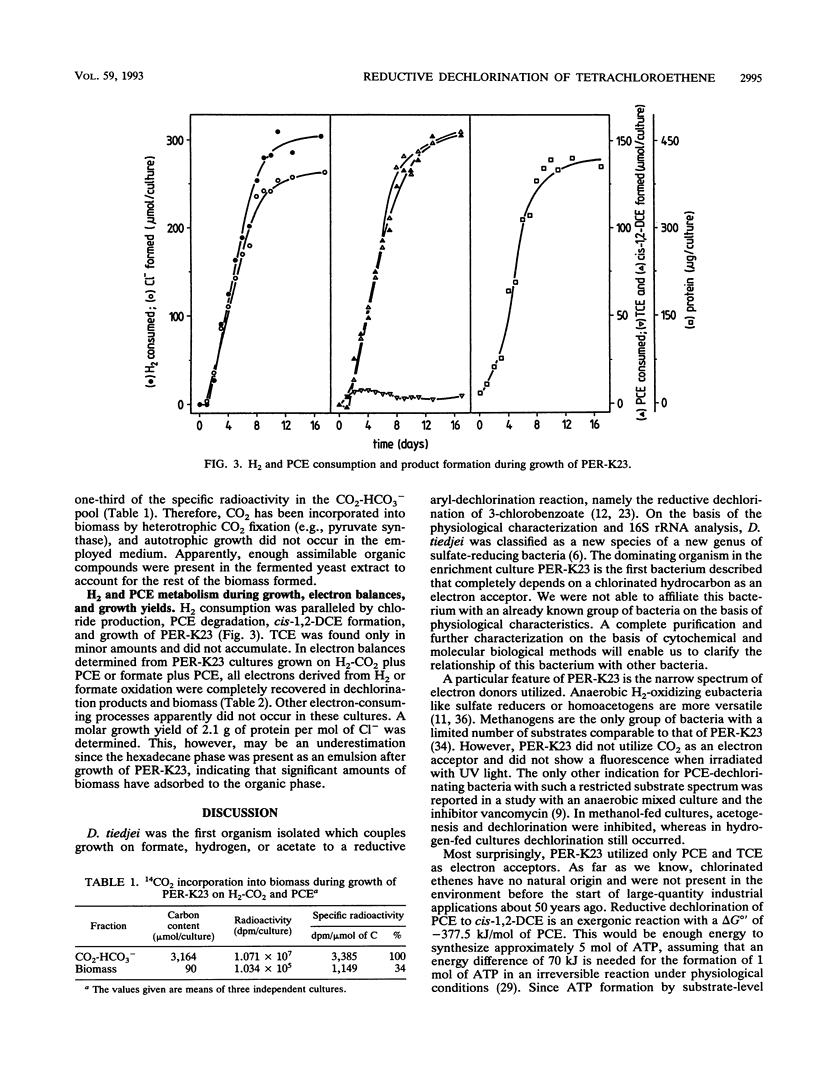


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belay N., Daniels L. Production of ethane, ethylene, and acetylene from halogenated hydrocarbons by methanogenic bacteria. Appl Environ Microbiol. 1987 Jul;53(7):1604–1610. doi: 10.1128/aem.53.7.1604-1610.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwer E. J., McCarty P. L. Transformations of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl Environ Microbiol. 1983 Apr;45(4):1286–1294. doi: 10.1128/aem.45.4.1286-1294.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano T. D., Gossett J. M., Zinder S. H. Hydrogen as an electron donor for dechlorination of tetrachloroethene by an anaerobic mixed culture. Appl Environ Microbiol. 1992 Nov;58(11):3622–3629. doi: 10.1128/aem.58.11.3622-3629.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano T. D., Gossett J. M., Zinder S. H. Reductive dechlorination of high concentrations of tetrachloroethene to ethene by an anaerobic enrichment culture in the absence of methanogenesis. Appl Environ Microbiol. 1991 Aug;57(8):2287–2292. doi: 10.1128/aem.57.8.2287-2292.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekert G., Konheiser U., Piechulla K., Thauer R. K. Nickel requirement and factor F430 content of methanogenic bacteria. J Bacteriol. 1981 Nov;148(2):459–464. doi: 10.1128/jb.148.2.459-464.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfing J. Reductive dechlorination of 3-chlorobenzoate is coupled to ATP production and growth in an anaerobic bacterium, strain DCB-1. Arch Microbiol. 1990;153(3):264–266. doi: 10.1007/BF00249079. [DOI] [PubMed] [Google Scholar]
- Dolfing J., Tiedje J. M. Growth yield increase linked to reductive dechlorination in a defined 3-chlorobenzoate degrading methanogenic coculture. Arch Microbiol. 1987;149(2):102–105. doi: 10.1007/BF00425073. [DOI] [PubMed] [Google Scholar]
- Fathepure B. Z., Boyd S. A. Dependence of tetrachloroethylene dechlorination on methanogenic substrate consumption by Methanosarcina sp. strain DCM. Appl Environ Microbiol. 1988 Dec;54(12):2976–2980. doi: 10.1128/aem.54.12.2976-2980.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathepure B. Z., Nengu J. P., Boyd S. A. Anaerobic bacteria that dechlorinate perchloroethene. Appl Environ Microbiol. 1987 Nov;53(11):2671–2674. doi: 10.1128/aem.53.11.2671-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D. L., Gossett J. M. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl Environ Microbiol. 1989 Sep;55(9):2144–2151. doi: 10.1128/aem.55.9.2144-2151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliger C., Schraa G., Stams A. J., Zehnder A. J. Enrichment and properties of an anaerobic mixed culture reductively dechlorinating 1,2,3-trichlorobenzene to 1,3-dichlorobenzene. Appl Environ Microbiol. 1992 May;58(5):1636–1644. doi: 10.1128/aem.58.5.1636-1644.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn W. W., Tiedje J. M. Strain DCB-1 conserves energy for growth from reductive dechlorination coupled to formate oxidation. Arch Microbiol. 1990;153(3):267–271. doi: 10.1007/BF00249080. [DOI] [PubMed] [Google Scholar]
- Scholz P. M., Kedem J., Sideman S., Beyar R., Weiss H. R. Effect of hyper- and hypovolaemia on regional myocardial oxygen consumption. Cardiovasc Res. 1990 Feb;24(2):81–86. doi: 10.1093/cvr/24.2.81. [DOI] [PubMed] [Google Scholar]
- Vogel T. M., McCarty P. L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985 May;49(5):1080–1083. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin W. P., Kotterman M. J., Posthumus M. A., Schraa G., Zehnder A. J. Complete biological reductive transformation of tetrachloroethene to ethane. Appl Environ Microbiol. 1992 Jun;58(6):1996–2000. doi: 10.1128/aem.58.6.1996-2000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]