Abstract
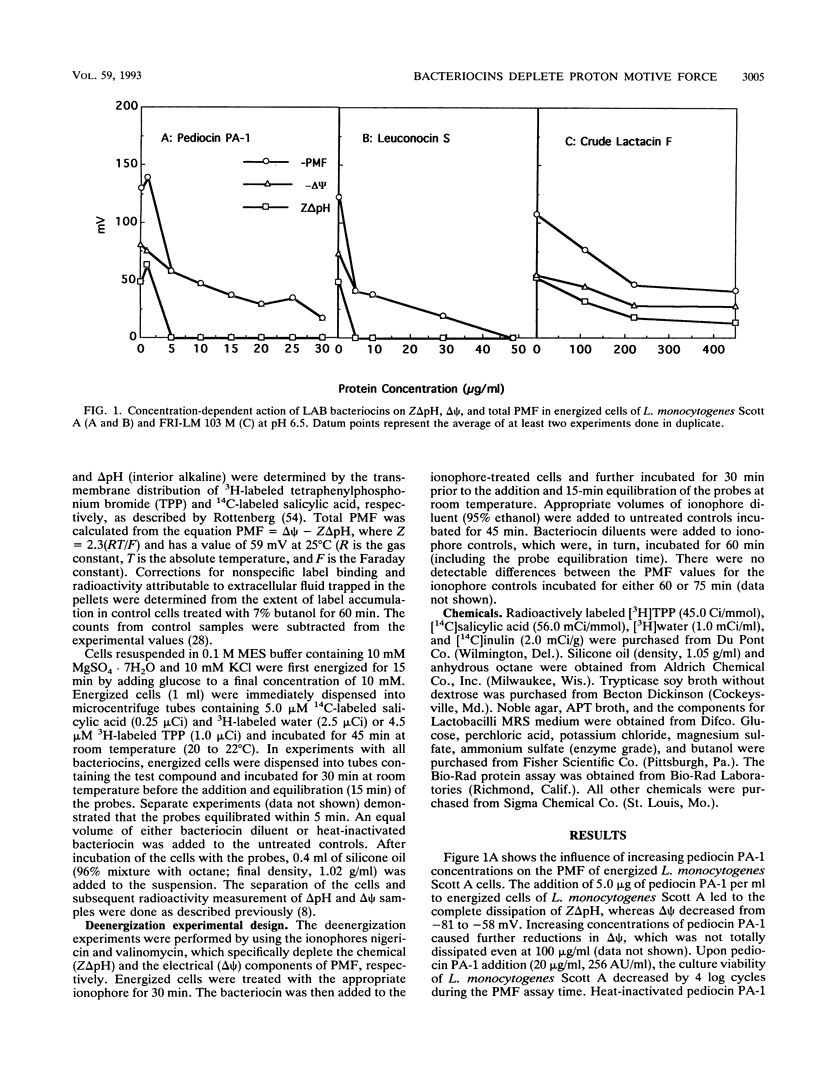
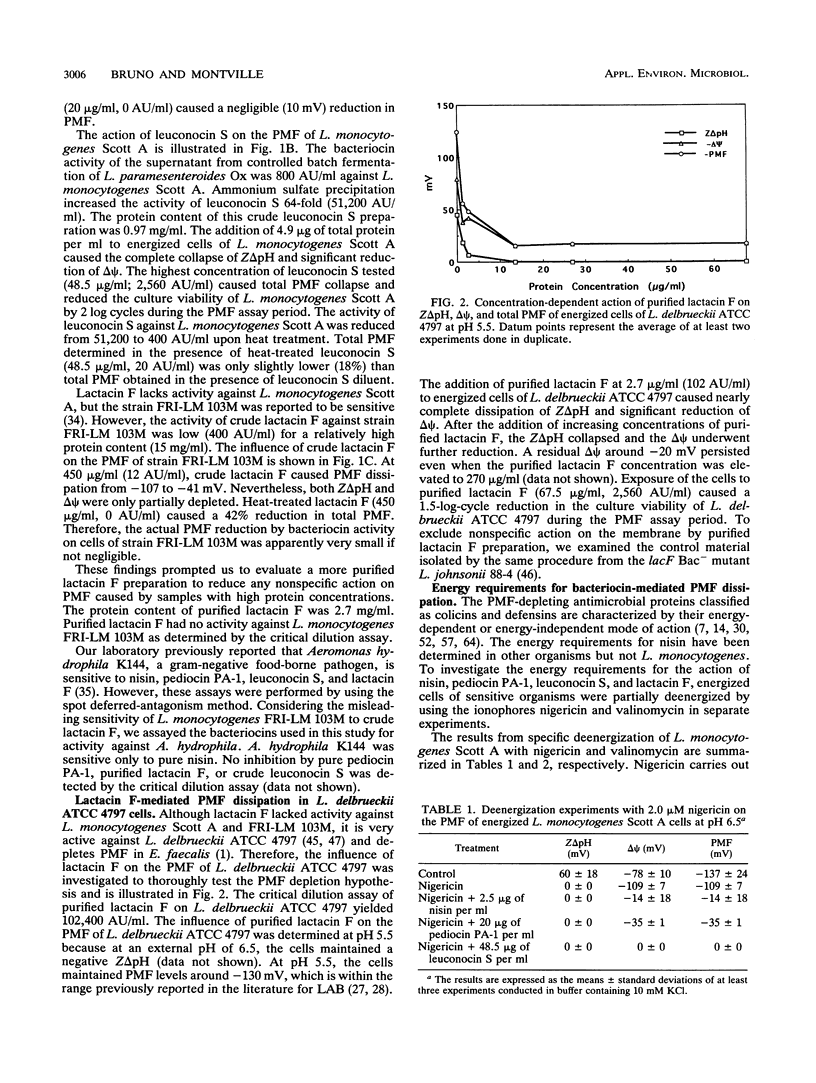
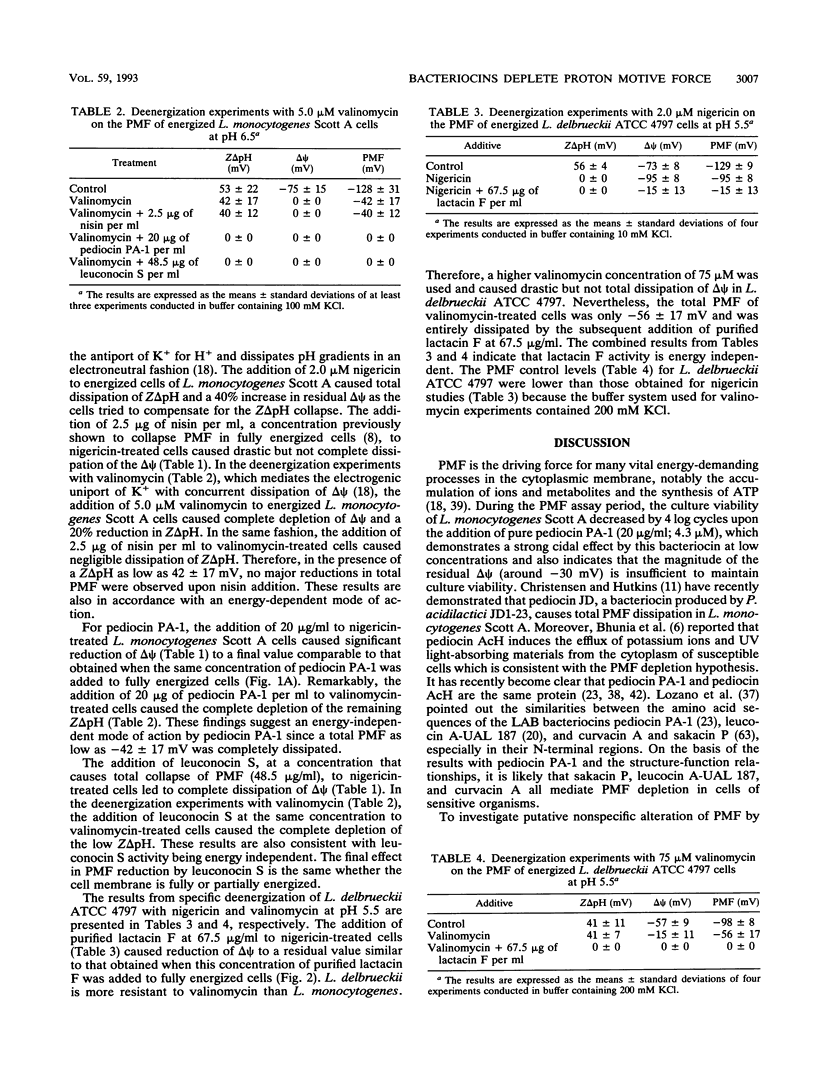
The influence of four bacteriocins from lactic acid bacteria on the proton motive force (PMF) of sensitive organisms was investigated. Pediocin PA-1 (20 μg/ml) and leuconocin S (48.5 μg/ml) mediated total or major PMF dissipation of energized Listeria monocytogenes Scott A cells in a concentration-dependent manner, as has been shown for nisin. Lactacin F (13.5 μg/ml) caused 87% PMF depletion of energized Lactobacillus delbrueckii ATCC 4797 cells, also in a concentration-dependent fashion. The energy requirements for the activity of these four bacteriocins were determined by using the ionophores nigericin and valinomycin to carry out partial and specific deenergization of the target organisms. Pediocin PA-1, leuconocin S, and lactacin F acted in an energy-independent manner, whereas the activity of nisin was confirmed to be energy dependent. These results together with published reports on other bacteriocins suggest that the bacteriocins of lactic acid bacteria share a common mechanism, the depletion of PMF.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S., Booth I. R. The use of valinomycin, nigericin and trichlorocarbanilide in control of the protonmotive force in Escherichia coli cells. Biochem J. 1983 Apr 15;212(1):105–112. doi: 10.1042/bj2120105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhunia A. K., Johnson M. C., Ray B. Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J Appl Bacteriol. 1988 Oct;65(4):261–268. doi: 10.1111/j.1365-2672.1988.tb01893.x. [DOI] [PubMed] [Google Scholar]
- Bourdineaud J. P., Boulanger P., Lazdunski C., Letellier L. In vivo properties of colicin A: channel activity is voltage dependent but translocation may be voltage independent. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1037–1041. doi: 10.1073/pnas.87.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M. E., Kaiser A., Montville T. J. Depletion of proton motive force by nisin in Listeria monocytogenes cells. Appl Environ Microbiol. 1992 Jul;58(7):2255–2259. doi: 10.1128/aem.58.7.2255-2259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman G. W., Banerjee S., Hansen J. N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J Biol Chem. 1988 Nov 5;263(31):16260–16266. [PubMed] [Google Scholar]
- Christensen D. P., Hutkins R. W. Collapse of the proton motive force in Listeria monocytogenes caused by a bacteriocin produced by Pediococcus acidilactici. Appl Environ Microbiol. 1992 Oct;58(10):3312–3315. doi: 10.1128/aem.58.10.3312-3315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Hall H. K. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J Bacteriol. 1991 Aug;173(16):5129–5135. doi: 10.1128/jb.173.16.5129-5135.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T., Benno Y., Yaeshima T., Mitsuoka T. Taxonomic study of the Lactobacillus acidophilus group, with recognition of Lactobacillus gallinarum sp. nov. and Lactobacillus johnsonii sp. nov. and synonymy of Lactobacillus acidophilus group A3 (Johnson et al. 1980) with the type strain of Lactobacillus amylovorus (Nakamura 1981). Int J Syst Bacteriol. 1992 Jul;42(3):487–491. doi: 10.1099/00207713-42-3-487. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Lehrer R. I. Defensins. Eur J Haematol. 1990 Jan;44(1):1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Gao F. H., Abee T., Konings W. N. Mechanism of action of the peptide antibiotic nisin in liposomes and cytochrome c oxidase-containing proteoliposomes. Appl Environ Microbiol. 1991 Aug;57(8):2164–2170. doi: 10.1128/aem.57.8.2164-2170.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross E., Morell J. L. The structure of nisin. J Am Chem Soc. 1971 Sep 8;93(18):4634–4635. doi: 10.1021/ja00747a073. [DOI] [PubMed] [Google Scholar]
- Hastings J. W., Sailer M., Johnson K., Roy K. L., Vederas J. C., Stiles M. E. Characterization of leucocin A-UAL 187 and cloning of the bacteriocin gene from Leuconostoc gelidum. J Bacteriol. 1991 Dec;173(23):7491–7500. doi: 10.1128/jb.173.23.7491-7500.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellingwerf K. J., Konings W. N. The energy flow in bacteria: the main free energy intermediates and their regulatory role. Adv Microb Physiol. 1985;26:125–154. doi: 10.1016/s0065-2911(08)60396-3. [DOI] [PubMed] [Google Scholar]
- Henderson J. T., Chopko A. L., van Wassenaar P. D. Purification and primary structure of pediocin PA-1 produced by Pediococcus acidilactici PAC-1.0. Arch Biochem Biophys. 1992 May 15;295(1):5–12. doi: 10.1016/0003-9861(92)90480-k. [DOI] [PubMed] [Google Scholar]
- Holck A., Axelsson L., Birkeland S. E., Aukrust T., Blom H. Purification and amino acid sequence of sakacin A, a bacteriocin from Lactobacillus sake Lb706. J Gen Microbiol. 1992 Dec;138(12):2715–2720. doi: 10.1099/00221287-138-12-2715. [DOI] [PubMed] [Google Scholar]
- Holo H., Nilssen O., Nes I. F. Lactococcin A, a new bacteriocin from Lactococcus lactis subsp. cremoris: isolation and characterization of the protein and its gene. J Bacteriol. 1991 Jun;173(12):3879–3887. doi: 10.1128/jb.173.12.3879-3887.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Héchard Y., Dérijard B., Letellier F., Cenatiempo Y. Characterization and purification of mesentericin Y105, an anti-Listeria bacteriocin from Leuconostoc mesenteroides. J Gen Microbiol. 1992 Dec;138(12):2725–2731. doi: 10.1099/00221287-138-12-2725. [DOI] [PubMed] [Google Scholar]
- Kaletta C., Entian K. D. Nisin, a peptide antibiotic: cloning and sequencing of the nisA gene and posttranslational processing of its peptide product. J Bacteriol. 1989 Mar;171(3):1597–1601. doi: 10.1128/jb.171.3.1597-1601.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Blanchard A. G., Metzger W. C. Proton motive force during growth of Streptococcus lactis cells. J Bacteriol. 1980 Jul;143(1):128–134. doi: 10.1128/jb.143.1.128-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaenhammer T. R. Bacteriocins of lactic acid bacteria. Biochimie. 1988 Mar;70(3):337–349. doi: 10.1016/0300-9084(88)90206-4. [DOI] [PubMed] [Google Scholar]
- Konisky J. Colicins and other bacteriocins with established modes of action. Annu Rev Microbiol. 1982;36:125–144. doi: 10.1146/annurev.mi.36.100182.001013. [DOI] [PubMed] [Google Scholar]
- Kordel M., Benz R., Sahl H. G. Mode of action of the staphylococcinlike peptide Pep 5: voltage-dependent depolarization of bacterial and artificial membranes. J Bacteriol. 1988 Jan;170(1):84–88. doi: 10.1128/jb.170.1.84-88.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordel M., Schüller F., Sahl H. G. Interaction of the pore forming-peptide antibiotics Pep 5, nisin and subtilin with non-energized liposomes. FEBS Lett. 1989 Feb 13;244(1):99–102. doi: 10.1016/0014-5793(89)81171-8. [DOI] [PubMed] [Google Scholar]
- Lewus C. B., Kaiser A., Montville T. J. Inhibition of food-borne bacterial pathogens by bacteriocins from lactic acid bacteria isolated from meat. Appl Environ Microbiol. 1991 Jun;57(6):1683–1688. doi: 10.1128/aem.57.6.1683-1688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewus C. B., Sun S., Montville T. J. Production of an Amylase-Sensitive Bacteriocin by an Atypical Leuconostoc paramesenteroides Strain. Appl Environ Microbiol. 1992 Jan;58(1):143–149. doi: 10.1128/aem.58.1.143-149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Hansen J. N. Enhancement of the chemical and antimicrobial properties of subtilin by site-directed mutagenesis. J Biol Chem. 1992 Dec 15;267(35):25078–25085. [PubMed] [Google Scholar]
- Marugg J. D., Gonzalez C. F., Kunka B. S., Ledeboer A. M., Pucci M. J., Toonen M. Y., Walker S. A., Zoetmulder L. C., Vandenbergh P. A. Cloning, expression, and nucleotide sequence of genes involved in production of pediocin PA-1, and bacteriocin from Pediococcus acidilactici PAC1.0. Appl Environ Microbiol. 1992 Aug;58(8):2360–2367. doi: 10.1128/aem.58.8.2360-2367.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Motlagh A. M., Bhunia A. K., Szostek F., Hansen T. R., Johnson M. C., Ray B. Nucleotide and amino acid sequence of pap-gene (pediocin AcH production) in Pediococcus acidilactici H. Lett Appl Microbiol. 1992 Aug;15(2):45–48. doi: 10.1111/j.1472-765x.1992.tb00721.x. [DOI] [PubMed] [Google Scholar]
- Mugikura S., Nishikawa M., Igarashi K., Kobayashi H. Maintenance of a neutral cytoplasmic pH is not obligatory for growth of Escherichia coli and Streptococcus faecalis at an alkaline pH. J Biochem. 1990 Jul;108(1):86–91. doi: 10.1093/oxfordjournals.jbchem.a123168. [DOI] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Conjugal Transfer of Plasmid-Encoded Determinants for Bacteriocin Production and Immunity in Lactobacillus acidophilus 88. Appl Environ Microbiol. 1987 Mar;53(3):553–560. doi: 10.1128/aem.53.3.553-560.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriana P. M., Klaenhammer T. R. Purification and partial characterization of lactacin F, a bacteriocin produced by Lactobacillus acidophilus 11088. Appl Environ Microbiol. 1991 Jan;57(1):114–121. doi: 10.1128/aem.57.1.114-121.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mørtvedt C. I., Nissen-Meyer J., Sletten K., Nes I. F. Purification and amino acid sequence of lactocin S, a bacteriocin produced by Lactobacillus sake L45. Appl Environ Microbiol. 1991 Jun;57(6):1829–1834. doi: 10.1128/aem.57.6.1829-1834.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto Lozano J. C., Meyer J. N., Sletten K., Peláz C., Nes I. F. Purification and amino acid sequence of a bacteriocin produced by Pediococcus acidilactici. J Gen Microbiol. 1992 Sep;138(9):1985–1990. doi: 10.1099/00221287-138-9-1985. [DOI] [PubMed] [Google Scholar]
- Nissen-Meyer J., Holo H., Håvarstein L. S., Sletten K., Nes I. F. A novel lactococcal bacteriocin whose activity depends on the complementary action of two peptides. J Bacteriol. 1992 Sep;174(17):5686–5692. doi: 10.1128/jb.174.17.5686-5692.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke A., Montville T. J. Bacteriocin-mediated inhibition of Clostridium botulinum spores by lactic acid bacteria at refrigeration and abuse temperatures. Appl Environ Microbiol. 1991 Dec;57(12):3423–3428. doi: 10.1128/aem.57.12.3423-3428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke A., Montville T. J. Nisin dissipates the proton motive force of the obligate anaerobe Clostridium sporogenes PA 3679. Appl Environ Microbiol. 1992 Aug;58(8):2463–2467. doi: 10.1128/aem.58.8.2463-2467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler U., Braun V., Wittmann-Liebold B., Benz R. Structural and functional properties of colicin B. J Biol Chem. 1986 Feb 25;261(6):2654–2659. [PubMed] [Google Scholar]
- Pucci M. J., Vedamuthu E. R., Kunka B. S., Vandenbergh P. A. Inhibition of Listeria monocytogenes by using bacteriocin PA-1 produced by Pediococcus acidilactici PAC 1.0. Appl Environ Microbiol. 1988 Oct;54(10):2349–2353. doi: 10.1128/aem.54.10.2349-2353.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Ruhr E., Sahl H. G. Mode of action of the peptide antibiotic nisin and influence on the membrane potential of whole cells and on cytoplasmic and artificial membrane vesicles. Antimicrob Agents Chemother. 1985 May;27(5):841–845. doi: 10.1128/aac.27.5.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahl H. G., Grossgarten M., Widger W. R., Cramer W. A., Brandis H. Structural similarities of the staphylococcin-like peptide Pep-5 to the peptide antibiotic nisin. Antimicrob Agents Chemother. 1985 May;27(5):836–840. doi: 10.1128/aac.27.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein S. J., Kagan B. L., Finkelstein A. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature. 1978 Nov 9;276(5684):159–163. doi: 10.1038/276159a0. [DOI] [PubMed] [Google Scholar]
- Schillinger U., Kaya M., Lücke F. K. Behaviour of Listeria monocytogenes in meat and its control by a bacteriocin-producing strain of Lactobacillus sake. J Appl Bacteriol. 1991 Jun;70(6):473–478. doi: 10.1111/j.1365-2672.1991.tb02743.x. [DOI] [PubMed] [Google Scholar]
- Schüller F., Benz R., Sahl H. G. The peptide antibiotic subtilin acts by formation of voltage-dependent multi-state pores in bacterial and artificial membranes. Eur J Biochem. 1989 Jun 1;182(1):181–186. doi: 10.1111/j.1432-1033.1989.tb14815.x. [DOI] [PubMed] [Google Scholar]
- Tagg J. R., Dajani A. S., Wannamaker L. W. Bacteriocins of gram-positive bacteria. Bacteriol Rev. 1976 Sep;40(3):722–756. doi: 10.1128/br.40.3.722-756.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda H., Konisky J. Effect of colicins Ia and E1 on ion permeability of liposomes. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6167–6171. doi: 10.1073/pnas.76.12.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venema K., Abee T., Haandrikman A. J., Leenhouts K. J., Kok J., Konings W. N., Venema G. Mode of Action of Lactococcin B, a Thiol-Activated Bacteriocin from Lactococcus lactis. Appl Environ Microbiol. 1993 Apr;59(4):1041–1048. doi: 10.1128/aem.59.4.1041-1048.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkowski K., Crandall A. D., Montville T. J. Inhibition of Listeria monocytogenes by Lactobacillus bavaricus MN in beef systems at refrigeration temperatures. Appl Environ Microbiol. 1993 Aug;59(8):2552–2557. doi: 10.1128/aem.59.8.2552-2557.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdel J. K., Ceglowski P., Dobrazański W. T. Mechanism of action of lactostrepcin 5, a bacteriocin produced by Streptococcus cremoris 202. Appl Environ Microbiol. 1985 Apr;49(4):969–974. doi: 10.1128/aem.49.4.969-974.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum M. J., Kok J., Venema G. Cloning, sequencing, and expression in Escherichia coli of lcnB, a third bacteriocin determinant from the lactococcal bacteriocin plasmid p9B4-6. Appl Environ Microbiol. 1992 Feb;58(2):572–577. doi: 10.1128/aem.58.2.572-577.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Belkum M. J., Kok J., Venema G., Holo H., Nes I. F., Konings W. N., Abee T. The bacteriocin lactococcin A specifically increases permeability of lactococcal cytoplasmic membranes in a voltage-independent, protein-mediated manner. J Bacteriol. 1991 Dec;173(24):7934–7941. doi: 10.1128/jb.173.24.7934-7941.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]



