Abstract
The conditioning of the pharmacological actions of cocaine with environmental stimuli is thought to be a critical factor in the long-term addictive potential of this drug. Cocaine-related stimuli may increase the likelihood of relapse by evoking drug craving, and brain-imaging studies have identified the amygdala and nucleus accumbens (NAcc) as putative neuroanatomical substrates for these effects of cocaine cues. To study the significance of environmental stimuli in the recovery of extinguished cocaine-seeking behavior, male Wistar rats were trained to associate discriminative stimuli (SΔs) with response-contingent availability of intravenous cocaine vs. saline. The rats then were subjected to repeated extinction sessions during which cocaine, saline, and the respective SΔs were withheld until the animals reached an extinction criterion of ≤4 responses over three consecutive sessions. Subsequent re-exposure to the cocaine SΔ, but not the nonreward SΔ, produced strong recovery of responding at the previously active lever in the absence of any further drug availability. The efficacy and behavioral selectivity of the cocaine SΔ remained unaltered throughout an 8-day test period. Exposure to the cocaine SΔ significantly increased dopamine efflux in the NAcc and amygdala as measured by intracranial microdialysis in a separate group of rats. Dopamine levels remained unaltered in the presence of the nonreward SΔ. The results demonstrate that cocaine-predictive stimuli elicit robust and persistent cocaine-seeking behavior, and that this effect may involve activation of dopamine transmission in the NAcc and amygdala.
The classical conditioning of the pharmacological actions of cocaine with environmental stimuli is thought to have an important role in the long-term addictive potential of this drug. Environmental cues repeatedly associated with the subjective effects of cocaine can elicit drug craving (1–6) and possibly, automatic behavioral responses (7, 8) that may lead to relapse in recovering cocaine addicts. Whereas the role of drug-related stimuli in motivating the resumption of drug use is not fully understood, such learned responses may be among the most important factors responsible for the high rates of relapse associated with cocaine and other drug addiction (9).
Consistent with the well-established conditioned reactivity to cocaine cues in humans, classically conditioned behavioral responses to cocaine can be readily elicited in animals (10–14). However, it has been more difficult to demonstrate motivating effects of stimuli conditioned to cocaine in animal models of relapse. Stimuli paired contiguously with cocaine infusions in self-administering rats can reinstate responding following extinction (15–17), but these stimuli often produce only weak and transient effects (16, 17), or fail to elicit cocaine-seeking behavior (18, 19). The lack of robust and enduring behavioral effects of cocaine cues in many “reinstatement” studies appears inconsistent with the presumed strength and persistence of the motivating effects of drug cues in humans.
An important consideration concerning the stimulus control of drug-seeking behavior involves the role of discriminative stimuli. Discriminative stimuli signal the availability of a reinforcer and thereby set the occasion to engage in behavior that brings the organism into contact with the reinforcing substance. Indeed, a condition often associated with drug craving in humans is cognitive awareness of drug availability (20). It has been argued, therefore, that the manner in which drug-associated contextual cues attain their incentive properties is likely to involve the predictive nature of these stimuli rather than only classically conditioned stimulus-response associations (21).
The present study sought to investigate the role of drug-associated discriminative stimuli in the addictive potential of cocaine, and to establish a behavioral model of “relapse” elicited by drug-related environmental stimuli. A secondary objective was to identify possible neurochemical substrates of cue-induced cocaine-seeking behavior. Brain-imaging studies in humans suggest a role for dopamine-rich brain regions, including the nucleus accumbens (NAcc) and amygdala in cue-induced cocaine craving (22–24). In animals, dopaminergic mechanisms in the NAcc and amygdala have been implicated in the unconditioned and conditioned behavioral effects of both drug and nondrug reinforcers (25–32). To test whether the conditioned behavioral effects of cocaine cues recruit dopamine (DA) transmission in these brain regions as well, the effects of a cocaine-associated discriminative stimulus on extracellular DA levels in the NAcc and amygdala were determined by microdialysis.
Materials and Methods
Subjects.
Male Wistar rats (Charles River Breeding Laboratories) weighing 250–300 g at the beginning of the experiment were used. Rats were housed in groups of two to three in a temperature-controlled (22°C) vivarium on a normal 12-h light/12-h dark cycle and had ad libitum access to food and water except during the initial stage of self-administration training. All procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Self-Administration Stations.
Rats were trained and tested in standard operant conditioning chambers located inside sound-attenuating cubicles (BRS/LVE, Laurel, MD). All chambers were equipped with two retractable levers. A house light was situated 22 cm above the food trough. A white cue light (24 W) was located 4 cm above each lever, and a house light was situated 22 cm above the food trough. Auditory stimuli consisted of a 70-dB white noise produced by a white-noise generator (Salk Institute) and presented via an 80-ohm speaker positioned in the center of the chamber front panel, or a 7-kHz, 70-dB intermittent tone generated by a tone source (Sonalert, Model SC628, 6–28 VDC, Mallory, CO) also located in the center of the front panel just above the speaker. Intravenous infusions were administered by a syringe pump (Razel, Stamford, CT) located outside the sound-attenuating cubicles and connected via a cannula connector (C3136 Plastics One, Roanoke, VA) to a chronic jugular catheter on the backs of the rats (33). Behavioral equipment and data collection were controlled by an IBM-compatible microcomputer.
Drugs.
Cocaine hydrochloride (National Institute on Drug Abuse, Bethesda, MD) was dissolved in sterile physiological saline at a concentration of 0.25 mg/0.1 ml. Drug or vehicle solution was infused at a volume of 0.1 ml over 4 s.
Self-Administration and Behavioral Testing Procedures.
All training and testing was conducted during the light phase of the light/dark cycle at the same time each day. To facilitate acquisition of cocaine self-administration, the rats were first trained to lever-press for food pellets. During this time, the animals were placed on a restricted diet (Purina rat chow, 15 g/day). The first session consisted of 30 min of noncontingent delivery of one 45-mg food pellet (Noyes, Lancaster, NH) every 15 s while the levers remained retracted. The next day, food pellets were made available response-contingently in daily 1-h sessions. Only the right lever was available, and each response resulted in delivery of one food pellet and 1-s illumination of the cue light above the lever. Once the animals had completed two sessions in which they earned 100 pellets each, food was made available again ad libitum.
Jugular catheter implantation.
The rats then were implanted with chronic silastic catheters in the right jugular vein under halothane (1.0–1.5%) anesthesia as described (33). Catheter patency was maintained by flushing with 0.1 ml of sterile saline solution containing 33.3 USP units/ml heparin before and after each self-administration session. Rats with compromised catheters were implanted with a new catheter in the contralateral jugular vein.
Cocaine self-administration and conditioning phase.
The purpose of these procedures was to train animals to intravenously self-administer cocaine while simultaneously establishing discriminative stimuli (SΔs) associated with, and predictive of, cocaine availability vs. nonavailability. Self-administration training began 7 days after surgery. During the first two days of this stage, sessions were initiated by extension of the right lever, at which time cocaine was made available for 2 h on a schedule of continuous reinforcement. After each injection, the lever remained inactive for 20 s to prevent accidental overdosing. This time-out (TO) period was signaled by illumination of the cue light above the lever. Starting on day 3, sessions were initiated by extension of both levers and concurrent presentation of a white noise that lasted throughout the session and served as a discriminative stimulus (S+) for cocaine availability. Only the right lever was active and when depressed, produced a cocaine infusion and illumination of the cue light during the 20-s TO period. Responses at the left, inactive lever were recorded but had no programmed consequences. Beginning on day 5, the self-administration procedure was changed from a single daily 2-h session to two consecutive 1-h sessions, separated by a 30-min period. A third daily 1-h session during which the cocaine solution was replaced with saline was introduced on day 7. This session was initiated by extension of both levers and simultaneous illumination of the house light which remained present for the duration of the 1-h period and served as a discriminative stimulus (S−) for cocaine nonavailability. Each response at the right lever produced an infusion of saline and presentation of an intermittent tone (7 kHz, 70 dB) as a TO cue for 20 s during which time the lever remained inactive. The rats then were placed on a “discrimination learning” regimen as follows: In three consecutive daily 1-h sessions, either cocaine or saline was available as the only infusion solution in an unpredictable sequence with the constraint that each training day included one saline and two cocaine sessions. Rats were removed from the self-administration chambers for 20–30 min between cocaine sessions, and 40–60 min between cocaine and saline sessions. During this phase, the cocaine S+ or S− continued to be present throughout cocaine or saline sessions, respectively, and drug or vehicle infusions continued to be followed by a signaled 20-s TO period. The SΔs were not turned off during the TO periods. Training was conducted daily until cocaine-reinforced responding stabilized (±10% over three consecutive training days) and rats ceased responding during saline sessions (≤4 responses during each of three successive sessions).
Extinction phase.
Each animal was placed on extinction conditions beginning one day after reaching the training criterion. During this stage, sessions began by extension of the levers without presentation of the discriminative stimuli. Responses at the previously active lever resulted in activation of the syringe pump motor but had no other programmed consequences. One daily extinction session was conducted lasting 1 h, until a criterion of ≤4 responses per session over three consecutive days was reached.
Reinstatement phase.
Reinstatement tests began one day after individual animals reached the extinction criterion. These tests lasted 1 h and were conducted once per day by exposing rats to the S+ or S− under conditions identical to the discrimination learning phase, except that cocaine or saline were not available. Rats were randomly assigned to testing with the S+ (Group 1) vs. S− (Group 2). In both conditions, presentation of the respective SΔ began simultaneously with extension of the levers and terminated with their retraction at the end of each session. As during self-administration training, responses at the active lever were followed by a 20-s signaled TO period. Responses at the inactive lever were recorded but had no programmed consequences. To further verify the behavioral selectivity of the S+ vs. S−, the cue conditions were reversed after the first 3 consecutive days of testing, and rats of group one were tested in the presence of the S− whereas rats of group two were tested in the presence of the S+. These tests were conducted 5 days after the initial 3-day reinstatement test period (i.e., on day 8 following completion of the extinction phase).
Intracranial Microdialysis.
Two additional groups of rats were designated for testing of the effects of the S+ vs. S− on the release of dopamine in the NAcc and amygdala.
Surgery.
Rats were stereotaxically implanted with a stainless steel guide cannula aimed at the NAcc or the region of the central/basolateral amygdala (AMY) under halothane (1.0–1.5%) anesthesia. The guide cannulae were lowered unilaterally toward the dorsal border of the dialysis site and secured with stainless steel skull screws and dental cement. With reference to bregma, the coordinates were A, +1.7 mm; M, ±1.4 mm; and V, −6.1 mm (NAcc) and A, −2.56 mm; M, ± 4.2 mm; and V, −7.8 mm (AMY) according to the atlas of Paxinos and Watson (34). The microdialysis sites were located between ventral coordinates V, −6.1 and V, −8.1 (NAcc) and V, −7.8 and V, −9.8 (AMY). Microdialysis guide cannulae and jugular catheters for intravenous self-administration were implanted in a single surgical session.
General microdialysis methods and procedures.
Guide cannulae and microdialysis probes (outer diameter, 300 μm) were constructed as described (35). The probes were perfused with artificial cerebrospinal fluid (149 mM NaCl/2.8 mM KCl/1.2 mM CaCl2/1.2 mM MgCl2/0.25 mM ascorbic acid/5.4 mM d-glucose/pH 7.2–7.4) at a rate of 0.2 μl/min by using a pulseless syringe pump (CMA/100; Bioanalytical Systems, West Lafayette, IN), and slowly inserted under brief halothane anesthesia (<5 min) 12–16 h before the start of testing. Dialysate was collected into 250-μl microfraction vials. Samples were immediately frozen on dry ice and stored at −70°C until assayed. Dialysate DA concentrations were measured by reverse-phase HPLC coupled with electrochemical detection. The mobile phase consisted of a 0.54 nM monobasic sodium phosphate buffer containing 0.9 mM 1-decanesulfonic acid/4.9 mM triethylamine/0.2 mM Na2EDTA/19% methanol/apparent pH 4.8, pumped (ISCO, Model 500) through the column at a rate of 20 μl/min. Dialysate samples were injected onto a 3-μ ODS-2 C-18 microbore column (100 mm × 1.0 mm) at a volume of 1 μl by using an electrically actuated valve (Model ECI4W1; Valco Instruments, Houston). Analytes were detected amperometrically (Princeton Applied Research, Model MP 1304) by dual glassy carbon working electrode (BAS, Model RE1, West Lafayette, IN). The applied potential was +700 mV (vs. Ag/AgCl). The detection limit for DA, defined by a signal/noise ratio of >3, was ≈0.2 nM.
Behavioral and test procedures.
Training and testing was conducted in operant conditioning chambers modified to accommodate the microdialysis perfusion system as described (27, 36). To habituate rats to the microdialysis procedures, the animals were repeatedly connected to an inactive perfusion system. All other behavioral procedures were identical to those described above. Microdialysis tests were performed during the first reinstatement test for each rat which was conducted either 1 or 2 days after completion of the extinction phase. Extracellular DA levels were monitored in 10-min fractions.
Verification of probe placements.
Brains were removed after sacrifice by 5% halothane and stored in 10% formaldehyde, and probe placements within the NAcc and AMY were verified by inspection of probe tracts under a light microscope. In all rats, at least 80% of the active region of the microdialysis membrane was located within the anatomical borders of the targeted site. Probe placements within the NAcc were distributed predominantly throughout the central accumbens, medial to the anterior commissure, but predominantly confined to the core region of the NAcc. Probes in the AMY traversed nearly the entire dorsal-ventral extent of this structure and were located between the basolateral and central nuclei, but also penetrated or made contact with the basomedial and anterior corticoid nuclei.
Data Analysis.
Behavioral data were analyzed for differences in the number of responses emitted by the S+ vs. S− reinstatement groups during the final three extinction sessions and the first three sessions of the reinstatement phase by mixed factorial ANOVA. After confirmation of significant effects or interactions by overall ANOVA, differences among groups or experimental phases were verified by Simple Effects ANOVA and/or Newman Keuls post hoc tests. Neurochemical data were analyzed for differences in dialysate DA concentrations between reinstatement (S+ vs. S−) conditions and brain regions (NAcc vs. AMY) by mixed factorial ANOVA, followed by Simple Effects ANOVA to determine differences between brain regions and reinstatement conditions.
Results
Cocaine Self-Administration and Conditioning Phase.
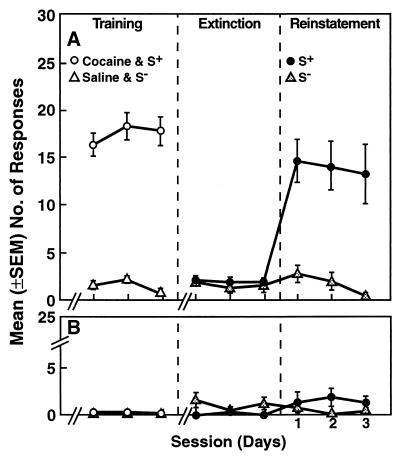
All rats began to develop stable rates of cocaine self-administration between 6–9 days of training without differences in responding during the first and second daily hour of cocaine availability. Mean responses during saline sessions decreased to less than five per session within three days. No differences were observed in the mean (± SEM) number of days required to reach the training criterion between the groups of rats designated for testing under the S+ (Group 1, n = 7; 16.4 ± 1.4) vs. S− (Group 2, n = 8; 15.5 ± 2.5) condition during the reinstatement phase. In addition, responding during the last three days of the training phase as well as during the two daily cocaine sessions was identical in these groups (Fig. 1 Left). This was reflected by the lack of significant main effects between reinstatement groups (F1,13 = 0.04; not significant, NS), training days (F2,26 = 2.13; NS), or interactions between reinstatement groups and daily sessions (F2,26 = 0.19; NS).
Figure 1.
Lever-press responses during self-administration training, extinction, and reinstatement sessions at the active (A) and inactive (B) lever. Training phase: Cocaine-reinforced responses during the final 3 days of the self-administration phase in rats (n = 15) trained to associate SΔs with the availability of intravenous cocaine (S+) vs. saline (S−). No differences were observed between responses during the first and second daily hour of cocaine availability, and responses for cocaine or saline between rats designated for testing under S+ vs. S− conditions during the initial 3 days of the reinstatement phase (see Results). The data were, therefore, collapsed across groups and daily cocaine sessions for the purpose of this illustration. Extinction phase: Extinction responses at criterion. The extinction criterion (≤4 responses per session over three consecutive days) was reached within 16.4 ± 3.8 days (averaged across rats designated for testing under the S+ vs. S− condition during the reinstatement phase). Reinstatement phase: Responses under the S+ (n = 7) and S− (n = 8) reinstatement conditions. Exposure to the S+-elicited significant recovery of responding in the absence of further drug availability. Responding in the presence of the S− remained at extinction levels. For statistical comparisons, see Results.
Extinction Phase.
Rats reached the extinction criterion after an average of 16.5 sessions. There was no difference in the mean (± SEM) number of days to extinction between rats designated for testing under the S+ (16.4 ± 3.8) vs. S− (16.6 ± 3.6) condition during the reinstatement phase. There was also no difference in the number of responses of these two groups (F1,13 = 0.42; NS) during the last three days of the extinction phase (Fig. 1 Center).
Reinstatement Phase.
Introduction of the S+ produced immediate recovery of responding with overall behavioral output identical to that during self-administration of cocaine. This effect persisted during the 3 days of the initial reinstatement testing phase (Fig. 1 Right). Responding at the inactive lever was negligible in both groups. Inspection of the response patterns of individual animals revealed that the response density in the presence of the S+ was greatest at the beginning of the sessions, but sustained responding was observed throughout the remainder of the sessions. The distribution of reinstatement responses was distinctly different from nonreinforced responding as measured during the first session of the extinction phase (data not shown). Differences in responding between the extinction and reinstatement phases (Fig. 2) were confirmed by main effects of reinstatement condition (F1,13 = 63.29; P < 0.0001) and experimental phase (F1,13 = 56.60; P < 0.0001) as well as a significant interaction between these factors (F1,12 = 46.58; P < 0.0001). Post hoc Simple Effects ANOVAs revealed significant differences between extinction and reinstatement responses of the S+ group (F1,13 = 34.65; P < 0.0001) and between reinstatement responses in the S+ vs. S− groups (F1,26 = 117.26; P < 0.0001). In addition, statistical comparison of cocaine-reinforced responses during the last 3 days of the training phase with responses during the first 3 days of the reinstatement phase in the S+ group indicated that the total number of responses in these experimental phases was identical (F5,30 = 0.53; NS).
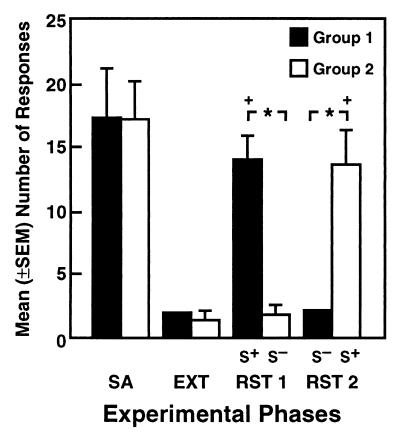
Figure 2.
Comparison of reinstatement responses after reversal of the SΔ cue conditions (RST 2) with responses during the self-administration training (SA), extinction (EXT), and reinstatement (RST 1) phases in rats assigned to the S+ (n = 5; Group 1) and S− (n = 7; Group 2) conditions during the initial reinstatement tests shown in Fig. 1. (Data for the SA, EXT, and RST 1 conditions represent averages across the last 3 days of the respective phase). Responding vs. nonresponding during the reinstatement tests was reliably altered as a function of the SΔ condition (*P < 0.0001 and +P < 0.01, significantly different from S− and extinction responses within each respective group).
Responses in rats exposed to the S− remained at extinction levels. Significant recovery of responding was, however, observed in these animals when they were subsequently tested in the presence of the S+. Conversely, rats of the S+ group that had shown a robust response reinstatement ceased responding when subsequently tested under the S− contingency (Fig. 2). Two animals developed health problems and one rat died during this experimental phase, reducing the sample size of the original S+ (Group 1) and S− (Group 2) groups to 5 and 7 rats, respectively. The dependence of responding vs. nonresponding on the SΔ conditions was confirmed by a significant difference in the mean number of responses between groups during the initial reinstatement phase (F1,38 = 16.79; P < 0.0001) and after reversal of the cue conditions (F1,38 = 15.09; P < 0.0001), as well as by significant differences in responding across the four experimental phases for the rats tested in the presence of the S+ (Group 1: F3,30 = 19.86; P < 0.0001) and S− (Group 2: F3,30 = 13.89; P < 0.0001) before reversal of the cue conditions [Simple Effects after significant interaction in overall mixed-factorial ANOVA (F3,30 = 12.03; P < 0.0001)]. Changes in responding as a result of reversing the S+ and S− cue conditions were further verified by one-way ANOVAs across experimental phases for Group 1 (F3,12 = 15.62; P < 0.0002) and Group 2 (F3,18 = 18.51; P < 0.0001) and subsequent Newman Keuls post hoc tests which confirmed significant differences in both groups between the S+ vs. S− (P < 0.01) and extinction (P < 0.01) conditions.
Dialysate Dopamine Levels.
Rats made fewer responses in the presence of the S+ during the microdialysis test compared with the first experiment which did not involve behavioral constraints associated with tethering and connection to the microdialysis perfusion system. Nonetheless, the number of responses emitted by rats tested in the presence of the S+ (7.9 ± 2.5; n = 11) was significantly greater (F1,17 = 6.44; P < 0.05) than in the S− condition (1.8 ± 0.6; n = 8), without differences between the NAcc and AMY groups as indicated by the lack of a main effect between groups (F1,17 = 1.1; NS) or interaction between stimulus conditions and groups (F1,17 = 0.1; NS). Data from two rats of the NAcc group were excluded from further data analysis because of large (>50%) erratic variations in basal DA efflux before the test (n = 1) or compromised tubing connections (n = 1). The differences in responding under the S+ (6.75 ± 1.42) vs. S− (2.4 ± 0.49) conditions remained significant after exclusion of these animals (F1,15 = 5.49; P < 0.05).
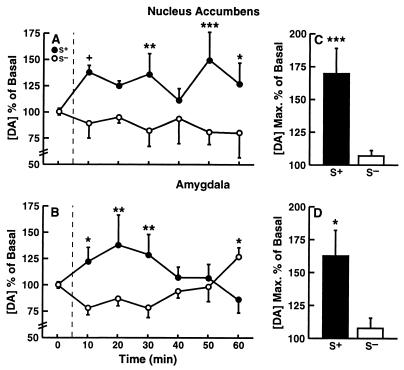
Mean (± SEM) basal dialysate DA concentrations in the NAcc across three 10-min sampling periods before presentation of the respective SΔs were 2.89 ± 0.60 nM (S+ group; n = 6) and 1.86 ± 0.77 nM (S− group; n = 4). The corresponding basal dialysate DA levels in the amygdala were 1.07 ± 0.74 nM (S+ group; n = 5) and 0.50 ± 0.03 nM (S− group; n = 4). Statistical analysis by unpaired Student's t tests indicated that there were no significant differences in basal DA efflux between rats assigned to the S+ vs. S− groups in either the NAcc (t8 = 1.07; NS) or AMY (t7 = 0.69; NS). DA efflux increased during exposure to the S+ in both brain regions (Fig. 3). Differences in dialysate DA concentrations (expressed as percent of baseline concentrations) in the S+ vs. S− condition were confirmed by significant main effects for reinstatement groups (F1,15 = 9.23; P < 0.01) and a significant interaction between reinstatement groups and sampling intervals (F8,120 = 4.30; P < 0.0001), without differences in cue-induced DA efflux between the two brain regions (F1,15 = 0.20; NS) or interactions between reinstatement groups and brain regions (F1,15 = 1.21; NS). Analysis of simple effects confirmed that dialysate DA levels were significantly changed from baseline only in the S+ (F8,120 = 4.48; P < 0.0001) but not the S− (F8,120 = 1.09; NS) condition. Simple effects analyses, conducted separately on the data of the NAcc and AMY groups after confirmation of significant reinstatement group × sampling interval interactions (NAcc: F8,56 = 3.54; P < 0.005; AMY: F8,64 = 3.29; P < 0.005), confirmed overall differences between DA levels in the S+ and S− groups in the NAcc (F8,64 = 4.13; P < 0.001) and AMY (F8,56 = 2.36; P < 0.05) as well as differences at individual sampling intervals (Fig. 3 A and B). Examination of the maximal increases (expressed as percentages of baseline) in accumbal DA efflux during the session in individual rats revealed peak elevations of 169.7 ± 18.96 in the S+ vs. 107.0 ± 4.12 in the S− condition (F1,15 = 9.8; P < 0.01). The corresponding peak increases in the AMY were 162 ± 6 vs. 107.75 ± 7.55 (F1,15 = 5.14; P < 0.05) in the S+ and S− conditions, respectively, (simple effects after overall ANOVA: F1,15 = 12.26; P < 0.005; Fig. 3 C and D).
Figure 3.
Mean (± SEM) dialysate DA levels in the nucleus accumbens (n = 10) and amygdala (n = 9) during exposure to the S+ and S− conditions. (A and B) The time course of dopaminergic activation during the 60-min reinstatement test in the two brain regions (***P < 0.001, **P < 0.1, and *P < 0.05; different from respective S− condition). (C and D) The maximal mean (± SEM) increase in DA efflux observed in each animal during the session (***P < 0.001 and *P < 0.05).
Discussion
The results confirm that discriminative stimuli associated with the availability and subjective effects of cocaine elicit robust cocaine-seeking behavior after prolonged extinction. The cue-induced recovery of responding persisted throughout 8 days of testing in the absence of further availability of the drug reinforcer. No increases in responding at the inactive lever were observed, ruling out nonspecific arousal as a factor in the response reinstatement. More importantly, lever-pressing in the presence of the S− did not increase over extinction levels, suggesting that the behavior of the animals was controlled selectively by the incentive properties of the cocaine S+. The behavioral significance of the cocaine S+ was further confirmed by the observation that rats initially tested in the presence of the S− showed complete recovery of responding when subsequently presented with the S+, whereas rats that had shown robust reinstatement ceased responding when later tested under S− conditions.
Given the established role of discriminative stimuli in the control of behavior, the response reinstatement by the cocaine S+ was not unexpected. Discriminative stimuli, by definition, signal the availability of a reinforcer and, thereby, elicit behavior directed at obtaining the respective reinforcer (37). Because extinction training for cocaine was conducted in the absence of the cocaine S+, its predictive reliability, and thus incentive salience, was allowed to remain intact. Consequently, the reintroduction of this stimulus was expected to elicit at least some resumption of cocaine-seeking behavior. Of significance, however, with regard to the presumed powerful role of cocaine-related environmental stimuli in “relapse” is that the cocaine S+ fully retained its efficacy and selectivity in controlling cocaine-seeking behavior over a 16.5 day mean abstinence period. In addition, exposure to the cocaine S+ did not merely elicit a transient behavioral response that extinguished when responses were not reinforced by cocaine, but in most rats produced sustained responding throughout the sessions. Strikingly, as well, the animals showed complete resistance to extinction to the effects of the cocaine S+ over 8 days of testing. These findings confirm a significant role of conditioning factors in the control of cocaine-seeking behavior, and by extension, support the hypothesis that learned responses to drug-related environmental stimuli are an important factor in chronic cocaine abuse and relapse. Moreover, in conjunction with earlier findings showing that discriminative stimuli can significantly retard the extinction of responding for psychostimulant reinforcers (38, 39), the results further implicate the incentive-motivational effect of such stimuli as an important factor in the control of compulsive drug-seeking behavior. Whereas the data confirmed that the response reinstatement by the cocaine S+ shows considerable resistance to extinction, it will, however, be important to systematically identify the factors that sustain the motivating effects of cocaine cues in future studies, particularly because this information may have direct implications for behavioral treatments aimed at extinguishing conditioned cocaine cue reactivity.
Exposure to the cocaine S+ increased DA efflux in the NAcc and amygdala. The magnitude of this effect corresponds well with increases in accumbal extracellular DA reported during anticipation of food, ethanol, saccharin, and sexual reward (27–29, 40, 41) as well as the degree of conditioned enhancement of low-dose cocaine effects on DA efflux by stimuli paired with a high cocaine dose (14). The present observations confirm that reward-predictive environmental stimuli elevate extracellular DA levels in the NAcc, but extend these findings to a role of DA transmission in the amygdala. Although in a recent report, a cocaine-associated conditioned stimulus did not alter DA efflux in the amygdala (19), this stimulus was also not effective in eliciting cocaine-seeking behavior. Therefore, the failure of the cocaine cue in the latter study to increase extracellular DA levels in the amygdala may have been the result of ineffective conditioning and does not contradict the present findings.
The anatomical placements and dimensions of the micro-dialysis probes preclude firm conclusions about the relative contribution of specific amygdaloid nuclei to the cue-induced increases in dialysate DA levels. Based on current understanding of the function of the amygdala in motivational and associative processes, both the central and basolateral nuclei are likely candidates. The central nucleus contains the highest density of DA terminals (42) and is thought to play a role in the modulation of the reinforcing and discriminative stimulus properties of cocaine (25, 26, 31) whereas the basolateral nucleus has been implicated in associative functions underlying many conditioned behavioral effects of psychostimulant drugs (43–45), including cue-induced recovery of cocaine-seeking behavior (32). Both of these nuclei were traversed by or within the most proximal sampling region of the microdialysis probes, and thus, are most likely the predominant sources of the observed changes in DA efflux.
With regard to the relevance of these findings for relapse in human drug addicts, the absence of a salient cocaine-related stimulus (i.e., the S+) during the extinction phase resembles the conditions that are typically in effect during withdrawal and abstinence in human drug addicts. Over the course of an individual's history of drug abuse, numerous stimuli become associated with the availability or subjective effects of the drug, and only few of these associations decay during treatment. Thus, the incentive salience of the cocaine S+ under the current experimental conditions was presumably similar to that of cocaine-associated contextual stimuli whose effects have not been effectively extinguished during abstinence in humans recovering from cocaine addiction. It is, however, important to emphasize that considerable quantitative differences exist between the learning history of rats under conditions such as in the present experiment, and the history of association learning in human drug abuse. Associations between drug-related stimuli and drug use in humans are usually formed on thousands of occasions over years of drug taking and, therefore, are likely to be far stronger than in animals that are exposed to only a limited number of such pairings. Thus, the present observations may, in effect, greatly underestimate the magnitude of cue-induced neurochemical responses in humans as well as their behavioral consequences.
Recent evidence from functional MRI and positron-emission tomography studies in humans show that cue-induced cocaine craving is associated with activation of dopamine-rich forebrain regions including the amygdala (22, 24, 46) and NAcc (47). These findings suggest that increased DA transmission in these brain regions may be one of the neurobiological mechanisms underlying cocaine craving associated with exposure to drug-related stimuli. The dopaminergic activation in the amygdala and NAcc by the cocaine S+ in the present study provides further support for this hypothesis. Additional support for a role for DA neurotransmission in conditioned cocaine-seeking behavior comes from findings that dopamine D1 receptor antagonists effectively block the response reinstatement induced by a cocaine-predictive SΔ (48, 49). However, systematic studies with site-specific injection of DA antagonists or selective lesioning approaches will be required to establish a specific involvement of dopaminergic mechanisms in the NAcc or amygdala in this effect. In this respect, very recent observations have implicated in particular the basolateral amygdala in cue-induced cocaine-seeking behavior. Functional ablation of the basolateral amygdala by local infusion of the sodium channel blocker tetrodotoxin attenuated cue-induced cocaine-seeking behavior in rats (32, 50), whereas electrical stimulation of the basolateral amygdala elicited reinstatement of extinguished cocaine-seeking behavior (51). In contrast, tetrodotoxin administration into the NAcc failed to inhibit conditioned drug-seeking, but did not interfere with responding reinforced directly by cocaine (50). Whereas these observations point toward an important involvement of the basolateral amygdala in conditioned cocaine-seeking behavior, the present results suggest that neural mechanisms in the NAcc may contribute to the behavioral effects of cocaine-related stimuli as well. It remains to be determined, however, whether drug-predictive stimuli engage dopaminergic mechanisms in the NAcc that are involved in anticipatory and attentional processes (52), or whether activation of DA neurotransmission in the NAcc reflects processes that have a more direct role in the control of behavior by external stimuli that are of motivational significance.
In conclusion, the results demonstrate that environmental stimuli previously predictive of cocaine availability reliably elicit drug-seeking behavior in rats, and that this effect may involve activation of dopamine neurons in the NAcc and amygdala. Moreover, the present behavioral procedures which generated behavior that was independent of the direct reinforcing actions of cocaine and highly resistant to extinction may provide an effective tool for the investigation of neurobiological mechanisms leading to drug-induced behavioral plasticity and vulnerability to relapse.
Acknowledgments
We thank Mike Arends for assistance with preparation of the manuscript. This is publication number 12263-NP from the Scripps Research Institute. This work was supported by National Institute on Drug Abuse Grant DA07348 (F.W.); C.S.M.-V. was supported in part by the APA Minority Fellowship Program in Neuroscience.
Abbreviations
- NAcc
nucleus accumbens
- SΔ
discriminative stimulus
- DA
dopamine
- TO
time-out period
- S+
discriminative stimulus for cocaine availability
- S−
discriminative stimulus for cocaine nonavailability
- AMY
amygdala, central/basolateral nucleus
- NS
not significant
References
- 1.Childress A R, Ehrman R N, McLellan A T, O'Brien C P. NIDA Research Monograph 81. Washington, DC: US Government Printing Office; 1988. pp. 74–80. [PubMed] [Google Scholar]
- 2.Ehrman R N, Robbins S J, Childress A R, O'Brien C P. Psychopharmacology. 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 3.Robbins S J, Ehrman R N, Childress A R, O'Brien C P. Addict Behav. 1997;22:157–167. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- 4.Kilgus M D, Pumariega A J. Southern Med J. 1994;87:1138–1140. doi: 10.1097/00007611-199411000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Satel S L, Krystal J H, Delgado P L, Kosten T R, Charney D S. Am J Psychiatr. 1995;152:778–783. doi: 10.1176/ajp.152.5.778. [DOI] [PubMed] [Google Scholar]
- 6.Wallace B C. J Subst Abuse Treat. 1989;6:95–106. doi: 10.1016/0740-5472(89)90036-6. [DOI] [PubMed] [Google Scholar]
- 7.Miller N S, Gold M S. Ann Clin Psychiatr. 1994;6:99–106. doi: 10.3109/10401239409148988. [DOI] [PubMed] [Google Scholar]
- 8.Tiffany S T, Carter B L. J Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien C P, Childress A R, Ehrman R, Robbins S J. J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 10.Tatum A L, Seevers M H. J Pharmacol Exp Ther. 1929;36:401–410. [Google Scholar]
- 11.Barr G A, Sharpless N S, Cooper S, Schiff S R. Life Sci. 1983;33:1341–1351. doi: 10.1016/0024-3205(83)90817-2. [DOI] [PubMed] [Google Scholar]
- 12.Brown E E, Fibiger H C. Neuroscience. 1992;48:621–629. doi: 10.1016/0306-4522(92)90406-r. [DOI] [PubMed] [Google Scholar]
- 13.Brown E E, Robertson G S, Fibiger H C. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontana D J, Post R M, Pert A. Brain Res. 1993;629:31–39. doi: 10.1016/0006-8993(93)90477-5. [DOI] [PubMed] [Google Scholar]
- 15.Meil W M, See R E. Behav Pharmacol. 1996;7:754–763. [PubMed] [Google Scholar]
- 16.Fuchs R A, Tran-Nguyen L T, Specio S E, Groff R S, Neisewander J L. Psychopharmacology (Berl) 1998;135:151–160. doi: 10.1007/s002130050496. [DOI] [PubMed] [Google Scholar]
- 17.deWit H, Stewart J. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 18.Weissenborn R, Yackey M, Koob G F, Weiss F. Drug Alcohol Depend. 1995;38:237–246. doi: 10.1016/0376-8716(95)01107-a. [DOI] [PubMed] [Google Scholar]
- 19.Tran-Nguyen L T, Fuchs R A, Coffey G P, Baker D A, O'Dell L E, Neisewander J L. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- 20.Meyer R E, Mirin S M. The Heroin Stimulus: Implications for a Theory of Addiction. New York: Plenum; 1979. [Google Scholar]
- 21.McFarland K, Ettenberg A. Psychopharmacology (Berl) 1997;131:86–92. doi: 10.1007/s002130050269. [DOI] [PubMed] [Google Scholar]
- 22.Grant S, London E D, Newlin D B, Villemagne V L, Liu X, Contoreggi C, Phillips R L, Kimes A S, Margolin A. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breiter H C, Gollub R L, Weisskoff R M, Kennedy D N, Makris N, Berke J D, Goodman J M, Kantor H L, Gastfriend D R, Riorden J P, et al. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- 24.Childress A R, Mozley P D, McElgin W, Fitzgerald J, Reivich M, O'Brien C P. Am J Psychiatr. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caine S B, Heinrichs S C, Coffin V L, Koob G F. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- 26.Hurd Y L, McGregor A, Ponten M. Eur J Neurosci. 1997;9:2541–2548. doi: 10.1111/j.1460-9568.1997.tb01683.x. [DOI] [PubMed] [Google Scholar]
- 27.Weiss F, Lorang M T, Bloom F E, Koob G F. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- 28.Fiorino D F, Coury A, Phillips A G. J Neurosci. 1997;17:4849–4855. doi: 10.1523/JNEUROSCI.17-12-04849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson C, Nomikos G G, Collu M, Fibiger H C. J Neurosci. 1995;15:5169–5178. doi: 10.1523/JNEUROSCI.15-07-05169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGregor A, Roberts D C. Brain Res. 1993;624:245–252. doi: 10.1016/0006-8993(93)90084-z. [DOI] [PubMed] [Google Scholar]
- 31.Callahan P M, Bryan S K, Cunningham K A. Pharmacol Biochem Behav. 1995;51:759–766. doi: 10.1016/0091-3057(95)00027-t. [DOI] [PubMed] [Google Scholar]
- 32.Meil W M, See R E. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- 33.Caine S B, Lintz R, Koob G F. In: Behavioral Neuroscience: A Practical Approach. Saghal A, editor. Oxford: University; 1993. pp. 117–143. [Google Scholar]
- 34.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd Ed. San Diego: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 35.Parsons L H, Justice J B., Jr J Neurochem. 1992;58:212–218. doi: 10.1111/j.1471-4159.1992.tb09298.x. [DOI] [PubMed] [Google Scholar]
- 36.Parsons L H, Koob G F, Weiss F. J Pharmacol Exp Ther. 1995;274:1182–1191. [PubMed] [Google Scholar]
- 37.Fantino E, Logan C A. The Experimental Analysis of Behavior: A Biological Perspective. San Francisco: Freeman; 1979. [Google Scholar]
- 38.Falk J L, Lau C E. Pharmacol Biochem Behav. 1995;50:71–75. doi: 10.1016/0091-3057(94)00256-i. [DOI] [PubMed] [Google Scholar]
- 39.Stretch R, Gerber G J, Wood S M. Can J Physiol Pharmacol. 1971;49:581–589. doi: 10.1139/y71-075. [DOI] [PubMed] [Google Scholar]
- 40.Katner S N, Kerr T M, Weiss F. Behav Pharmacol. 1996;7:669–674. [PubMed] [Google Scholar]
- 41.Gonzales R A, Weiss F. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben-Ari Y, Zigmond R E, Moore K E. Brain Res. 1975;87:96–101. doi: 10.1016/0006-8993(75)90786-6. [DOI] [PubMed] [Google Scholar]
- 43.Whitelaw R B, Markou A, Robbins T W, Everitt B J. Psychopharmacology (Berl) 1996;127:213–224. [PubMed] [Google Scholar]
- 44.Cador M, Robbins T W, Everitt B J. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- 45.Hiroi N, White N M. J Neurosci. 1991;11:2107–2116. doi: 10.1523/JNEUROSCI.11-07-02107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garavan H, Bloom A S, Sperry L L, Pankiewicz J, Ward D, Risinger C, Salm T, Stein E A. National Institute on Drug Abuse Research Monograph Series 179. Washington, DC: US Government Printing Office; 1988. p. 108. (abstr.). [Google Scholar]
- 47.Childress A R, Mozley D, Fitzgerald J, Reivich M, Jaggi J, O'Brien C P. Soc Neurosci Abstr. 1995;21:1956. [Google Scholar]
- 48.Weiss F. Cocaine and a Changing Brain. Washington, DC: National Institute of Health; 1999. , Publication No. 99–4382, pp. 37–42. [Google Scholar]
- 49.Weiss F, Ciccocioppo R. Behav Pharmacol. 1999;10,Suppl. 1:S99. (abstr.). [Google Scholar]
- 50.Grimm J W, See R E. Soc Neurosci Abstr. 1999;25(1):1298. [Google Scholar]
- 51.Haves R J, Vorel S R, Liu X, Spector J, Lachman H, Gardner E L. Soc Neurosci Abstr. 1999;25(1):559. [Google Scholar]
- 52.Spanagel R, Weiss F. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]